A vertebrate RNA-binding protein Fox-1 regulates tissue-specific splicing via the pentanucleotide GCAUG
- PMID: 12574126
- PMCID: PMC145449
- DOI: 10.1093/emboj/cdg089
A vertebrate RNA-binding protein Fox-1 regulates tissue-specific splicing via the pentanucleotide GCAUG
Abstract
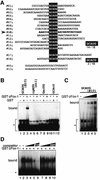
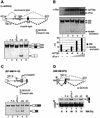
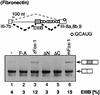
Alternative splicing is one of the central mechanisms that regulate eukaryotic gene expression. Here we report a tissue-specific RNA-binding protein, Fox-1, which regulates alternative splicing in vertebrates. Fox-1 bound specifically to a pentanucleotide GCAUG in vitro. In zebrafish and mouse, fox-1 is expressed in heart and skeletal muscles. As candidates for muscle-specific targets of Fox-1, we considered two genes, the human mitochondrial ATP synthase gamma-subunit gene (F1gamma) and the rat alpha-actinin gene, because their primary transcripts contain several copies of GCAUG. In transfection experiments, Fox-1 induced muscle-specific exon skipping of the F1gamma gene via binding to GCAUG sequences upstream of the regulated exon. Fox-1 also regulated mutually exclusive splicing of the alpha-actinin gene, antagonizing the repressive effect of polypyrimidine tract-binding protein (PTB). It has been reported that GCAUG is essential for the alternative splicing regulation of several genes including fibronectin. We found that Fox-1 promoted inclusion of the fibronectin EIIIB exon. Thus, we conclude that Fox-1 plays key roles in both positive and negative regulation of tissue-specific splicing via GCAUG.
Figures


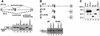
References
-
- Black D.L. (2000) Protein diversity from alternative splicing: a challenge for bioinformatics and post-genome biology. Cell, 103, 367–370. - PubMed
-
- Burd C.G. and Dreyfuss,G. (1994) Conserved structures and diversity of functions of RNA-binding proteins. Science, 265, 615–621. - PubMed
-
- Charlet B.N., Logan,P., Singh,G. and Cooper,T.A. (2002) Dynamic antagonism between ETR-3 and PTB regulates cell type-specific alternative splicing. Mol. Cell, 9, 649–658. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

