E1 initiator DNA binding specificity is unmasked by selective inhibition of non-specific DNA binding
- PMID: 12574131
- PMCID: PMC145451
- DOI: 10.1093/emboj/cdg091
E1 initiator DNA binding specificity is unmasked by selective inhibition of non-specific DNA binding
Abstract
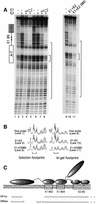
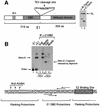
Initiator proteins are critical components of the DNA replication machinery and mark the site of initiation. This activity probably requires highly selective DNA binding; however, many initiators display modest specificity in vitro. We demonstrate that low specificity of the papillomavirus E1 initiator results from the presence of a non-specific DNA-binding activity, involved in melting, which masks the specificity intrinsic to the E1 DNA-binding domain. The viral factor E2 restores specificity through a physical interaction with E1 that suppresses non-specific binding. We propose that this arrangement, where one DNA-binding activity tethers the initiator to ori while another alters DNA structure, is a characteristic of other viral and cellular initiator proteins. This arrangement would provide an explanation for the low selectivity observed for DNA binding by initiator proteins.
Figures





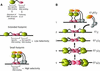
Similar articles
-
The E1 initiator recognizes multiple overlapping sites in the papillomavirus origin of DNA replication.J Virol. 2001 Jan;75(1):292-302. doi: 10.1128/JVI.75.1.292-302.2001. J Virol. 2001. PMID: 11119599 Free PMC article.
-
Characterization of the DNA-binding domain of the bovine papillomavirus replication initiator E1.J Virol. 1998 Apr;72(4):2567-76. doi: 10.1128/JVI.72.4.2567-2576.1998. J Virol. 1998. PMID: 9525573 Free PMC article.
-
Common determinants in DNA melting and helicase-catalysed DNA unwinding by papillomavirus replication protein E1.Nucleic Acids Res. 2006 May 31;34(10):3008-19. doi: 10.1093/nar/gkl384. Print 2006. Nucleic Acids Res. 2006. PMID: 16738139 Free PMC article.
-
Papillomavirus E1 proteins: form, function, and features.Virus Genes. 2002 Jun;24(3):275-90. doi: 10.1023/a:1015336817836. Virus Genes. 2002. PMID: 12086149 Review.
-
Initiation of DNA replication: lessons from viral initiator proteins.Nat Rev Mol Cell Biol. 2003 Oct;4(10):777-85. doi: 10.1038/nrm1226. Nat Rev Mol Cell Biol. 2003. PMID: 14504622 Review.
Cited by
-
A non-sequence-specific DNA binding mode of RAG1 is inhibited by RAG2.J Mol Biol. 2009 Apr 3;387(3):744-58. doi: 10.1016/j.jmb.2009.02.020. Epub 2009 Feb 20. J Mol Biol. 2009. PMID: 19232525 Free PMC article.
-
Analysis of chromatin attachment and partitioning functions of bovine papillomavirus type 1 E2 protein.J Virol. 2004 Feb;78(4):2100-13. doi: 10.1128/jvi.78.4.2100-2113.2004. J Virol. 2004. PMID: 14747575 Free PMC article.
-
Replication and partitioning of papillomavirus genomes.Adv Virus Res. 2008;72:155-205. doi: 10.1016/S0065-3527(08)00404-1. Adv Virus Res. 2008. PMID: 19081491 Free PMC article. Review.
-
Effective formation of the segregation-competent complex determines successful partitioning of the bovine papillomavirus genome during cell division.J Virol. 2010 Nov;84(21):11175-88. doi: 10.1128/JVI.01366-10. Epub 2010 Sep 1. J Virol. 2010. PMID: 20810736 Free PMC article.
-
The papillomavirus E2 proteins.Virology. 2013 Oct;445(1-2):57-79. doi: 10.1016/j.virol.2013.06.006. Epub 2013 Jul 10. Virology. 2013. PMID: 23849793 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

