p53 is a chromatin accessibility factor for nucleotide excision repair of DNA damage
- PMID: 12574133
- PMCID: PMC145442
- DOI: 10.1093/emboj/cdg082
p53 is a chromatin accessibility factor for nucleotide excision repair of DNA damage
Abstract
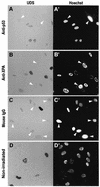
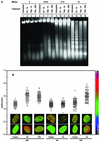
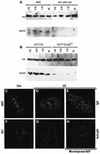
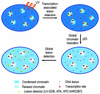
One of the longest standing problems in DNA repair is how cells relax chromatin in order to make DNA lesions accessible for global nucleotide excision repair (NER). Since chromatin has to be relaxed for efficient lesion detection, the key question is whether chromatin relaxation precedes lesion detection or vice versa. Chromatin accessibility factors have been proposed but not yet identified. Here we show that p53 acts as a chromatin accessibility factor, mediating UV-induced global chromatin relaxation. Using localized subnuclear UV irradiation, we demonstrate that chromatin relaxation is extended over the whole nucleus and that this process requires p53. We show that the sequence for initiation of global NER is as follows: transcription-associated lesion detection; p53-mediated global chromatin relaxation; and global lesion detection. The tumour suppressor p53 is crucial for genomic stability, a role partially explained by its pro-apoptotic capacity. We demonstrate here that p53 is also a fundamental component of DNA repair, playing a direct role in rectifying DNA damage.
Figures






Similar articles
-
Remodelling chromatin on a global scale: a novel protective function of p53.Carcinogenesis. 2004 Sep;25(9):1551-7. doi: 10.1093/carcin/bgh212. Epub 2004 Jul 1. Carcinogenesis. 2004. PMID: 15231688 Review.
-
The novel tumor suppressor p33ING2 enhances nucleotide excision repair via inducement of histone H4 acetylation and chromatin relaxation.Cancer Res. 2006 Feb 15;66(4):1906-11. doi: 10.1158/0008-5472.CAN-05-3444. Cancer Res. 2006. PMID: 16488987
-
Tumor suppressor p53 dependent recruitment of nucleotide excision repair factors XPC and TFIIH to DNA damage.DNA Repair (Amst). 2003 May 13;2(5):483-99. doi: 10.1016/s1568-7864(03)00002-8. DNA Repair (Amst). 2003. PMID: 12713809
-
p53 responsive nucleotide excision repair gene products p48 and XPC, but not p53, localize to sites of UV-irradiation-induced DNA damage, in vivo.Carcinogenesis. 2003 May;24(5):843-50. doi: 10.1093/carcin/bgg031. Carcinogenesis. 2003. PMID: 12771027
-
E2F1 and p53 transcription factors as accessory factors for nucleotide excision repair.Int J Mol Sci. 2012 Oct 19;13(10):13554-68. doi: 10.3390/ijms131013554. Int J Mol Sci. 2012. PMID: 23202967 Free PMC article. Review.
Cited by
-
p38 MAPK- and Akt-mediated p300 phosphorylation regulates its degradation to facilitate nucleotide excision repair.Nucleic Acids Res. 2013 Feb 1;41(3):1722-33. doi: 10.1093/nar/gks1312. Epub 2012 Dec 28. Nucleic Acids Res. 2013. PMID: 23275565 Free PMC article.
-
Oncogenic viral protein HPV E7 up-regulates the SIRT1 longevity protein in human cervical cancer cells.Aging (Albany NY). 2009 Mar 2;1(3):316-27. doi: 10.18632/aging.100028. Aging (Albany NY). 2009. PMID: 20157519 Free PMC article.
-
Rapid recruitment of p53 to DNA damage sites directs DNA repair choice and integrity.Proc Natl Acad Sci U S A. 2022 Mar 8;119(10):e2113233119. doi: 10.1073/pnas.2113233119. Epub 2022 Mar 2. Proc Natl Acad Sci U S A. 2022. PMID: 35235448 Free PMC article.
-
Interaction of p21(CDKN1A) with PCNA regulates the histone acetyltransferase activity of p300 in nucleotide excision repair.Nucleic Acids Res. 2008 Mar;36(5):1713-22. doi: 10.1093/nar/gkn014. Epub 2008 Feb 7. Nucleic Acids Res. 2008. PMID: 18263614 Free PMC article.
-
DNA multiphoton absorption generates localized damage for studying repair dynamics in live cells.Biophys J. 2011 Nov 2;101(9):2294-303. doi: 10.1016/j.bpj.2011.09.031. Epub 2011 Nov 1. Biophys J. 2011. PMID: 22067170 Free PMC article.
References
-
- Ait-Si-Ali S. et al. (2000) CBP/p300 histone acetyl-transferase activity is important for the G1/S transition. Oncogene, 19, 2430–2437. - PubMed
-
- Benhamou S. and Sarasin,A. (2000) Variability in nucleotide excision repair and cancer risk: a review. Mutat. Res., 462, 149–158. - PubMed
-
- Bunz F., Dutriaux,A., Lengauer,C., Waldman,T., Zhou,S., Brown,J.P., Sedivy,J.M., Kinzler,K.W. and Vogelstein,B. (1998) Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science, 282, 1497–1501. - PubMed
-
- Christians F.C. and Hanawalt,P.C. (1992) Inhibition of transcription and strand-specific DNA repair by α-amanitin in Chinese hamster ovary cells. Mutat. Res., 274, 93–101. - PubMed
-
- Cleaver J.E. and Thomas,G.H. (1981) Measurement of unscheduled DNA synthesis by autoradiography. In Friedberg,E.C. and Hanawalt,P.C. (eds), DNA Repair: A Laboratory Manual of Research Procedures. Vol I, part B. Marcel Dekker, New York, NY, pp. 277–287.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

