Use of a phage-based assay for phenotypic detection of mycobacteria directly from sputum
- PMID: 12574267
- PMCID: PMC149652
- DOI: 10.1128/JCM.41.2.680-688.2003
Use of a phage-based assay for phenotypic detection of mycobacteria directly from sputum
Abstract
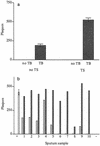
The phage amplified biologically assay is a new method for rapid and low-cost phenotypic determination of the drug sensitivities of Mycobacterium tuberculosis isolates and the detection of viable organisms in patient specimens. Infection of slowly growing mycobacteria with phage (phage D29) was followed by chemical virucide destruction of extracellular phage. Infected mycobacteria were mixed in culture with rapidly growing sensor cells, which the phage can also infect; i.e., lytic amplification of phage occurs. The aims of the present study were to optimize the speed and sensitivity of the assay and reduce its cost for developing countries by using an M. tuberculosis-spiked sputum model with (i). identification of inhibitory components of sputum and optimization of decontamination methods; (ii). simplification of the washing and development steps; (iii). reduction of the use of high-cost components, e.g., oleate-albumin-dextrose-catalase (OADC) supplement; and (iv). optimization of virucide treatment. The following results were obtained. (i). An inhibitory factor in sputum which could be removed by treatment of the sample with sodium dodecyl sulfate or NaOH decontamination was identified. (ii). A microcentrifuge-based approach with thixotropic silica as a bedding and resuspension agent was developed as an alternative to conventional centrifugation medium exchange. The yield was increased 228-fold, with increased speed and reduced cost. (iii). At present, after extracellular inactivation of phage, the ferrous ammonium sulfate (FAS) virucide is sequestered by dilution with an expensive supplement, OADC. Sodium citrate with calcium chloride was found to be a cost-effective after treatment with the FAS protectant and offered greater protection than OADC. Kinetic-lysis experiments indicated that an infection time of 1 to 3 h prior to FAS addition was optimal. (iv). Amplification of the signal (which corresponded to the burst size) was shown by allowing lysis prior to plating in a spiked medium model (up to 20-fold) and a spiked sputum model (up to 10-fold). A liquid culture detection method capable of detecting approximately 60 viable M. tuberculosis organisms in 1 ml of sputum was developed. Taken together, these improvements support the routine application of the assay to sputum specimens.
Figures



Similar articles
-
Phage lysin to control the overgrowth of normal flora in processed sputum samples for the rapid and sensitive detection of Mycobacterium tuberculosis by luciferase reporter phage assay.BMC Infect Dis. 2013 Jan 28;13:44. doi: 10.1186/1471-2334-13-44. BMC Infect Dis. 2013. PMID: 23356428 Free PMC article.
-
Comparison of amplified Q beta replicase and PCR assays for detection of Mycobacterium tuberculosis.J Clin Microbiol. 1995 Apr;33(4):860-7. doi: 10.1128/jcm.33.4.860-867.1995. J Clin Microbiol. 1995. PMID: 7540627 Free PMC article.
-
Phage cocktail to control the exponential growth of normal flora in processed sputum specimens grown overnight in liquid medium for rapid TB diagnosis.J Microbiol Methods. 2007 Mar;68(3):536-42. doi: 10.1016/j.mimet.2006.10.015. Epub 2006 Dec 13. J Microbiol Methods. 2007. PMID: 17173989
-
Inactivation of mycobacteriophage D29 using ferrous ammonium sulphate as a tool for the detection of viable Mycobacterium smegmatis and M. tuberculosis.Res Microbiol. 1998 Jul-Aug;149(7):487-95. doi: 10.1016/s0923-2508(98)80003-x. Res Microbiol. 1998. PMID: 9766200
-
Methods of sputum decontamination with emphasis on local tuberculosis laboratories.Afr J Med Med Sci. 2011 Mar;40(1):5-14. Afr J Med Med Sci. 2011. PMID: 21834256 Review.
Cited by
-
Synergistic Antimicrobial Interaction between Honey and Phage against Escherichia coli Biofilms.Front Microbiol. 2017 Dec 8;8:2407. doi: 10.3389/fmicb.2017.02407. eCollection 2017. Front Microbiol. 2017. PMID: 29276503 Free PMC article.
-
Phage Amplification Assay for Detection of Mycobacterial Infection: A Review.Microorganisms. 2021 Jan 23;9(2):237. doi: 10.3390/microorganisms9020237. Microorganisms. 2021. PMID: 33498792 Free PMC article. Review.
-
Generation of a novel nucleic acid-based reporter system to detect phenotypic susceptibility to antibiotics in Mycobacterium tuberculosis.mBio. 2012 Mar 13;3(2):e00312-11. doi: 10.1128/mBio.00312-11. Print 2012. mBio. 2012. PMID: 22415006 Free PMC article.
-
Application of bacteriophages for detection of foodborne pathogens.Bacteriophage. 2014 Jan 1;4(1):e28137. doi: 10.4161/bact.28137. Epub 2014 Feb 7. Bacteriophage. 2014. PMID: 24533229 Free PMC article. Review.
-
In-house, simple & economical phage technique for rapid detection of rifampicin, isoniazid, ethambutol, streptomycin & ciprofloxacin drug resistance using Mycobacterium tuberculosis isolates.Indian J Med Res. 2012 May;135(5):783-7. Indian J Med Res. 2012. PMID: 22771613 Free PMC article.
References
-
- Carrière, C., P. F. Riska, O. Zimhony, J. Kriakov, S. Bardarov, J. Burns, J. Chan, and W. R. Jacobs. 1997. Conditionally replicating luciferase reporter phages: improved sensitivity for rapid detection and assessment of drug susceptibility of Mycobacterium tuberculosis. J. Clin. Microbiol. 35:3232-3239. - PMC - PubMed
-
- Chatterjee, S., M. Mitra, and S. K. Das Gupta. 2000. A high yielding mutant of mycobacteriophage L1 and its application as a diagnostic tool. FEMS Microbiol. Lett. 188:47-53. - PubMed
-
- Collins, C. H., J. M. Grange, and M. D. Yates. 1997. Tuberculosis bacteriology: organisation and practice. Butterworth Heinemann, Oxford, England.
-
- David, H. L., S. Clavel, and F. Clement. 1979. Absorption and growth of the bacteriophage D29 in selected mycobacteria. Ann. Virol. 131:167-184.
-
- Drobniewski, F. A., A. Pablos-Méndez, and M. C. Raviglione. 1997. Epidemiology of tuberculosis in the world. Semin. Respir. Crit. Care Med 18:419-429.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

