AFLMP1 encodes an antigenic cel wall protein in Aspergillus flavus
- PMID: 12574298
- PMCID: PMC149704
- DOI: 10.1128/JCM.41.2.845-850.2003
AFLMP1 encodes an antigenic cel wall protein in Aspergillus flavus
Abstract
We have previously reported the cloning and characterization of the MP1 gene in Penicillium marneffei and the AFMP1 gene in Aspergillus fumigatus and their use for serodiagnosis of penicilliosis and aspergilloma and invasive aspergillosis, respectively. In this study, we describe the cloning of the AFLMP1 gene, which encodes the homologous antigenic cell wall protein in Aspergillus flavus, the most common Aspergillus species associated with human disease in our locality and in other Asian countries and the second most common Aspergillus species associated with human disease in Western countries. AFLMP1 codes for a protein, Aflmp1p, of 273 amino acid residues, with a few sequence features that are present in Mp1p and Afmp1p, the homologous antigenic cell wall proteins in P. marneffei and A. fumigatus, respectively, as well as several other cell wall proteins of Saccharomyces cerevisiae and Candida albicans. It contains a serine- and threonine-rich region for O glycosylation, a signal peptide, and a putative glycosylphosphatidylinositol attachment signal sequence. Specific anti-Aflmp1p antibody was generated with recombinant Aflmp1p protein purified from Escherichia coli to allow further characterization of Aflmp1p. Indirect immunofluorescence analysis indicated that Aflmp1p is present on the surface of the hyphae of A. flavus. Finally, it was observed that patients with aspergilloma and invasive aspergillosis due to A. flavus develop a specific antibody response against Aflmp1p. This suggested that the recombinant protein and its antibody may be useful for serodiagnosis in patients with aspergilloma or invasive aspergillosis, and the protein may represent a good cell surface target for host humoral immunity.
Figures


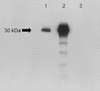
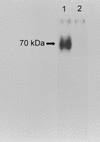


References
-
- Caras, I. W., and G. N. Weddell. 1989. Signal peptide for protein secretion directing glycophospholipid membrane anchor attachment. Science 243:1196-1198. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

