Fibroblast growth factor receptor 3 signaling regulates the onset of oligodendrocyte terminal differentiation
- PMID: 12574417
- PMCID: PMC6741926
- DOI: 10.1523/JNEUROSCI.23-03-00883.2003
Fibroblast growth factor receptor 3 signaling regulates the onset of oligodendrocyte terminal differentiation
Abstract
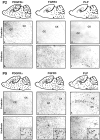
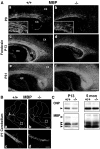
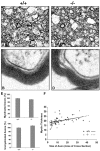
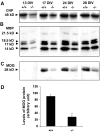
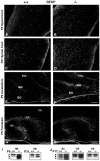
Fibroblast growth factor receptor (FGFR) signaling is essential for nervous system development. We have shown that, in the normal postnatal brain, the spatial and temporal expression pattern of FGFR3 parallels the appearance of differentiated oligodendrocytes and that in culture FGFR3 is expressed maximally at the critical stage in the lineage at which oligodendrocyte late progenitors (Pro-OLs) enter terminal differentiation. Therefore, FGFR3 expression is positioned ideally to have an impact on oligodendrocyte differentiation. In support of this we show that, during the onset and active phase of myelination in FGFR3-deficient mice, there are reduced numbers of differentiated oligodendrocytes in the forebrain, cerebellum, hindbrain, and spinal cord. Furthermore, myelination is delayed in parallel. Delay of oligodendrocyte differentiation also is observed in primary cell culture from this mutant. On the other hand, no differences are observed in the survival or proliferation of oligodendrocyte progenitors. This suggests that the decrease in the number of differentiated oligodendrocytes is attributable to a delay in the timing of their differentiation process. Astrocytes also express FGFR3, and in mice lacking FGFR3 there is an enhancement of the astrocytic marker glial fibrillary acidic protein expression in a region-specific manner. Thus our findings suggest that there are cell type- and region-specific functions for FGFR3 signaling and in particular emphasize a prominent role for FGFR3 as part of a system regulating the onset of oligodendrocyte terminal differentiation.
Figures




References
-
- Albrecht PJ, Dahl JP, Stoltzfus OK, Levenson R, Levison SW. Ciliary neurotrophic factor activates spinal cord astrocytes, stimulating their production and release of fibroblast growth factor-2, to increase motor neuron survival. Exp Neurol. 2002;173:46–62. - PubMed
-
- Asai T, Wanaka A, Kato H, Masana Y, Seo M, Tohyama M. Differential expression of two members of FGF receptor gene family, FGFR1 and FGFR2 mRNA, in the adult rat central nervous system. Brain Res Mol Brain Res. 1993;17:174–178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
