Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats
- PMID: 12574433
- PMCID: PMC6741915
- DOI: 10.1523/JNEUROSCI.23-03-01026.2003
Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats
Abstract
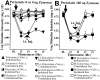
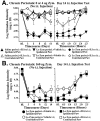
Mirror-image allodynia is a mysterious phenomenon that occurs in association with many clinical pain syndromes. Allodynia refers to pain in response to light touch/pressure stimuli, which normally are perceived as innocuous. Mirror-image allodynia arises from the healthy body region contralateral to the actual site of trauma/inflammation. Virtually nothing is known about the mechanisms underlying such pain. A recently developed animal model of inflammatory neuropathy reliably produces mirror-image allodynia, thus allowing this pain phenomenon to be analyzed. In this sciatic inflammatory neuropathy (SIN) model, decreased response threshold to tactile stimuli (mechanical allodynia) develops in rats after microinjection of immune activators around one healthy sciatic nerve at mid-thigh level. Low level immune activation produces unilateral allodynia ipsilateral to the site of sciatic inflammation; more intense immune activation produces bilateral (ipsilateral + mirror image) allodynia. The present studies demonstrate that both ipsilateral and mirror-image SIN-induced allodynias are (1) reversed by intrathecal (peri-spinal) delivery of fluorocitrate, a glial metabolic inhibitor; (2) prevented and reversed by intrathecal CNI-1493, an inhibitor of p38 mitogen-activated kinases implicated in proinflammatory cytokine production and signaling; and (3) prevented or reversed by intrathecal proinflammatory cytokine antagonists specific for interleukin-1, tumor necrosis factor, or interleukin-6. Reversal of ipsilateral and mirror-image allodynias was rapid and complete even when SIN was maintained constantly for 2 weeks before proinflammatory cytokine antagonist administration. These results provide the first evidence that ipsilateral and mirror-image inflammatory neuropathy pain are created both acutely and chronically through glial and proinflammatory cytokine actions.
Figures





References
-
- Aicher SA, Sharma S, Cheng PY, Pickel VM. The N-methyl-d-aspartate (NMDA) receptor is postsynaptic to substance P-containing axon terminals in the rat superficial dorsal horn. Brain Res. 1997;772:71–81. - PubMed
-
- Aloisi AM, Porro CA, Cavazzuti M, Baraldi P, Carli G. “Mirror pain” in the formalin test: behavioral and 2-deoxyglucose studies. Pain. 1993;55:267–273. - PubMed
-
- Arruda JL, Rutkowski MD, Sweitzer SM, DeLeo JA. Antibody and IgG attenuates mechanical allodynia in a mononeuropathy model in the rat: potential role of immune modulation in neuropathic pain. Brain Res. 2000;879:216–225. - PubMed
-
- Baron R. Peripheral neuropathic pain: from mechanisms to symptoms. Clin J Pain. 2000;16(Suppl 2):S12–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
