Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes
- PMID: 12574519
- PMCID: PMC151323
- DOI: 10.1073/pnas.0336132100
Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes
Abstract
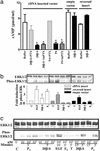
The structures of membrane receptors mediating rapid, nongenomic actions of steroids have not been identified. We describe the cloning of a cDNA from spotted seatrout ovaries encoding a protein that satisfies the following seven criteria for its designation as a steroid membrane receptor: plausible structure, tissue specificity, cellular distribution, steroid binding, signal transduction, hormonal regulation, and biological relevance. For plausible structure, computer modeling predicts that the protein has seven transmembrane domains, typical of G protein-coupled receptors. The mRNA (4.0 kb) is only detected in the brain and reproductive tissues on Northern blots. Antisera only detect the protein (40 kDa) in plasma membranes of reproductive tissues. The recombinant protein produced in an Escherichia coli expression system has a high affinity (K(d) = 30 nM), saturable, displaceable, single binding site specific for progestins. Progestins alter signal transduction pathways, activating mitogen-activated protein kinase and inhibiting adenylyl cyclase, in a transfected mammalian cell line. Inhibition of adenylyl cyclase is pertussis toxin sensitive, suggesting the receptor may be coupled to an inhibitory G protein. Progestins and gonadotropin up-regulate both mRNA and protein levels in seatrout ovaries. Changes in receptor abundance in response to hormones and at various stages of oocyte development, its probable coupling to an inhibitory G protein and inhibition of progestin induction of oocyte maturation upon microinjection of antisense oligonucleotides are consistent with the identity of the receptor as an intermediary in oocyte maturation. These characteristics suggest the fish protein is a membrane progestin receptor mediating a "nonclassical" action of progestins to induce oocyte maturation in fish.
Figures






Comment in
-
The further redefining of steroid-mediated signaling.Proc Natl Acad Sci U S A. 2003 Mar 4;100(5):2168-70. doi: 10.1073/pnas.0530224100. Epub 2003 Feb 26. Proc Natl Acad Sci U S A. 2003. PMID: 12606724 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

