Ca2+ phase waves: a basis for cellular pacemaking and long-range synchronicity in the guinea-pig gastric pylorus
- PMID: 12576498
- PMCID: PMC2342787
- DOI: 10.1113/jphysiol.2002.033720
Ca2+ phase waves: a basis for cellular pacemaking and long-range synchronicity in the guinea-pig gastric pylorus
Abstract
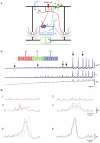
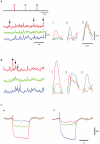
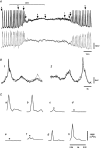
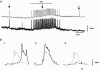
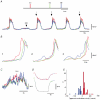
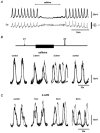
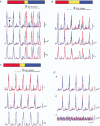
Ca2+ imaging and multiple microelectrode recording procedures were used to investigate a slow wave-like electrical rhythmicity in single bundle strips from the circular muscle layer of the guinea-pig gastric pylorus. The 'slow waves' (SWs) consisted of a pacemaker and regenerative component, with both potentials composed of more elementary events variously termed spontaneous transient depolarizations (STDs) or unitary potentials. STDs and SW pacemaker and regenerative potentials exhibited associated local and distributed Ca2+ transients, respectively. Ca2+ transients were often larger in cellular regions that exhibited higher basal Ca2+ indicator-associated fluorescence, typical of regions likely to contain intramuscular interstitial cells of Cajal (ICCIM). The emergence of rhythmicity arose through entrainment of STDs resulting in pacemaker Ca2+ transients and potentials, events that exhibited considerable spatial synchronicity. Application of ACh to strips exhibiting weak rhythmicity caused marked enhancement of SW synchronicity. SWs and underlying Ca2+ increases exhibited very high 'apparent conduction velocities' ('CVs') orders of magnitude greater than for sequentially conducting Ca2+ waves. Central interruption of either intercellular connectivity or inositol 1,4,5-trisphosphate receptor (IP3R)-mediated store Ca2+ release in strips caused SWs at the two ends to run independently of each other, consistent with a coupled oscillator-based mechanism. Central inhibition of stores required much wider regions of blockade than inhibition of connectivity indicating that stores were voltage-coupled. Simulations, made using a conventional store array model but now including depolarization coupled to IP3R-mediated Ca2+ release, predicted the experimental findings. The linkage between membrane voltage and Ca2+ release provides a means for stores to interact as strongly coupled oscillators, resulting in the emergence of Ca2+ phase waves and associated pacemaker potentials. This distributed pacemaker triggers regenerative Ca2+ release and resultant SWs.
Figures






Similar articles
-
Role of calcium stores and membrane voltage in the generation of slow wave action potentials in guinea-pig gastric pylorus.J Physiol. 2000 Apr 1;524 Pt 1(Pt 1):245-65. doi: 10.1111/j.1469-7793.2000.00245.x. J Physiol. 2000. PMID: 10747196 Free PMC article.
-
Generation and propagation of gastric slow waves.Clin Exp Pharmacol Physiol. 2010 Apr;37(4):516-24. doi: 10.1111/j.1440-1681.2009.05331.x. Epub 2009 Nov 23. Clin Exp Pharmacol Physiol. 2010. PMID: 19930430 Review.
-
Pacemaking through Ca2+ stores interacting as coupled oscillators via membrane depolarization.Biophys J. 2007 Jun 1;92(11):3843-61. doi: 10.1529/biophysj.106.095687. Epub 2007 Mar 9. Biophys J. 2007. PMID: 17351003 Free PMC article.
-
Spontaneous electrical and Ca2+ signals in typical and atypical smooth muscle cells and interstitial cell of Cajal-like cells of mouse renal pelvis.J Physiol. 2007 Sep 15;583(Pt 3):1049-68. doi: 10.1113/jphysiol.2007.137034. Epub 2007 Jul 26. J Physiol. 2007. PMID: 17656432 Free PMC article.
-
Electrophysiological properties of gastric pacemaker potentials.J Smooth Muscle Res. 2003 Oct;39(5):163-73. doi: 10.1540/jsmr.39.163. J Smooth Muscle Res. 2003. PMID: 14695027 Review.
Cited by
-
Phasic contractions of the mouse vagina and cervix at different phases of the estrus cycle and during late pregnancy.PLoS One. 2014 Oct 22;9(10):e111307. doi: 10.1371/journal.pone.0111307. eCollection 2014. PLoS One. 2014. PMID: 25337931 Free PMC article.
-
Ca2+ dynamics in interstitial cells: foundational mechanisms for the motor patterns in the gastrointestinal tract.Physiol Rev. 2024 Jan 1;104(1):329-398. doi: 10.1152/physrev.00036.2022. Epub 2023 Aug 10. Physiol Rev. 2024. PMID: 37561138 Free PMC article. Review.
-
Calcium events in smooth muscles and their interstitial cells; physiological roles of sparks.J Physiol. 2006 Jan 1;570(Pt 1):5-11. doi: 10.1113/jphysiol.2005.095604. Epub 2005 Sep 29. J Physiol. 2006. PMID: 16195319 Free PMC article. Review.
-
Luminal Mg2+, a key factor controlling RYR2-mediated Ca2+ release: cytoplasmic and luminal regulation modeled in a tetrameric channel.J Gen Physiol. 2008 Oct;132(4):429-46. doi: 10.1085/jgp.200810001. J Gen Physiol. 2008. PMID: 18824590 Free PMC article.
-
Expression and function of a T-type Ca2+ conductance in interstitial cells of Cajal of the murine small intestine.Am J Physiol Cell Physiol. 2014 Apr 1;306(7):C705-13. doi: 10.1152/ajpcell.00390.2013. Epub 2014 Jan 29. Am J Physiol Cell Physiol. 2014. PMID: 24477235 Free PMC article.
References
-
- Allbritton NL, Meyer T, Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 1992;258:1812–1815. - PubMed
-
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. - PubMed
-
- Best L, Bolton TB. Depolarisation of guinea-pig visceral smooth muscle causes hydrolysis of inositol phospholipids. Naunyn Schmiedebergs Arch Pharmacol. 1986;333:78–82. - PubMed
-
- Bootman MD, Berridge MJ, Lipp P. Cooking with calcium: the recipes for composing global signals from elementary events. Cell. 1997;91:367–373. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

