Distinct contributions of small and large conductance Ca2+-activated K+ channels to rat Purkinje neuron function
- PMID: 12576503
- PMCID: PMC2342800
- DOI: 10.1113/jphysiol.2002.027854
Distinct contributions of small and large conductance Ca2+-activated K+ channels to rat Purkinje neuron function
Abstract
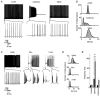
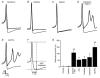
The cerebellum is important for many aspects of behaviour, from posture maintenance and goal-oriented reaching movements to timing tasks and certain forms of learning. In every case, information flowing through the cerebellum passes through Purkinje neurons, which receive input from the two primary cerebellar afferents and generate continuous streams of action potentials that constitute the sole output from the cerebellar cortex to the deep nuclei. The tonic firing behaviour observed in Purkinje neurons in vivo is maintained in brain slices even when synaptic inputs are blocked, suggesting that Purkinje neuron activity relies to a significant extent on intrinsic conductances. Previous research has suggested that the interplay between Ca2+ currents and Ca2+-activated K+ channels (KCa channels) is important for Purkinje cell activity, but how many different KCa channel types are present and what each channel type contributes to cell behaviour remains unclear. In order to better understand the ionic mechanisms that control the behaviour of these neurons, we investigated the effects of different Ca2+ channel and KCa channel antagonists on Purkinje neurons in acute slices of rat cerebellum. Our data show that Ca2+ entering through P-type voltage-gated Ca2+ channels activates both small-conductance (SK) and large-conductance (BK) KCa channels. SK channels play a role in setting the intrinsic firing frequency, while BK channels regulate action potential shape and may contribute to the unique climbing fibre response.
Figures
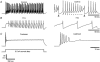
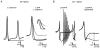


Similar articles
-
Iberiotoxin-sensitive large conductance Ca2+ -dependent K+ (BK) channels regulate the spike configuration in the burst firing of cerebellar Purkinje neurons.Brain Res. 2008 May 30;1212:1-8. doi: 10.1016/j.brainres.2008.03.030. Epub 2008 Mar 27. Brain Res. 2008. PMID: 18439989
-
Developmental regulation of small-conductance Ca2+-activated K+ channel expression and function in rat Purkinje neurons.J Neurosci. 2002 Jun 1;22(11):4456-67. doi: 10.1523/JNEUROSCI.22-11-04456.2002. J Neurosci. 2002. PMID: 12040053 Free PMC article.
-
Ionic mechanisms of burst firing in dissociated Purkinje neurons.J Neurosci. 2003 Oct 22;23(29):9650-63. doi: 10.1523/JNEUROSCI.23-29-09650.2003. J Neurosci. 2003. PMID: 14573545 Free PMC article.
-
Small conductance Ca2+-activated K+ channels as targets of CNS drug development.Curr Drug Targets CNS Neurol Disord. 2004 Jun;3(3):161-7. doi: 10.2174/1568007043337472. Curr Drug Targets CNS Neurol Disord. 2004. PMID: 15180477 Review.
-
KCa 2.2 (KCNN2): A physiologically and therapeutically important potassium channel.J Neurosci Res. 2023 Nov;101(11):1699-1710. doi: 10.1002/jnr.25233. Epub 2023 Jul 19. J Neurosci Res. 2023. PMID: 37466411 Free PMC article. Review.
Cited by
-
Reduced expression of the Ca(2+) transporter protein PMCA2 slows Ca(2+) dynamics in mouse cerebellar Purkinje neurones and alters the precision of motor coordination.J Physiol. 2010 Mar 15;588(Pt 6):907-22. doi: 10.1113/jphysiol.2009.182196. Epub 2010 Jan 18. J Physiol. 2010. PMID: 20083513 Free PMC article.
-
Ca2+- and Voltage-Activated K+ (BK) Channels in the Nervous System: One Gene, a Myriad of Physiological Functions.Int J Mol Sci. 2023 Feb 8;24(4):3407. doi: 10.3390/ijms24043407. Int J Mol Sci. 2023. PMID: 36834817 Free PMC article. Review.
-
BK channels control cerebellar Purkinje and Golgi cell rhythmicity in vivo.PLoS One. 2009 Nov 24;4(11):e7991. doi: 10.1371/journal.pone.0007991. PLoS One. 2009. PMID: 19956720 Free PMC article.
-
Hyperactivity of Purkinje cell and motor deficits in C9orf72 knockout mice.Mol Cell Neurosci. 2022 Jul;121:103756. doi: 10.1016/j.mcn.2022.103756. Epub 2022 Jul 16. Mol Cell Neurosci. 2022. PMID: 35843530 Free PMC article.
-
Encoding of oscillations by axonal bursts in inferior olive neurons.Neuron. 2009 May 14;62(3):388-99. doi: 10.1016/j.neuron.2009.03.023. Neuron. 2009. PMID: 19447094 Free PMC article.
References
-
- Blatz AL, Magleby KL. Single apamin-blocked Ca-activated K+ channels of small conductance in cultured rat skeletal muscle. Nature. 1986;323:718–720. - PubMed
-
- Chung YH, Shin C, Park KH, Cha CI. Immunohistochemical study on the distribution of neuronal voltage-gated calcium channels in the rat cerebellum. Brain Res. 2000;865:278–282. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

