Defective presentation of the CD1d1-restricted natural Va14Ja18 NKT lymphocyte antigen caused by beta-D-glucosylceramide synthase deficiency
- PMID: 12576547
- PMCID: PMC149922
- DOI: 10.1073/pnas.0430327100
Defective presentation of the CD1d1-restricted natural Va14Ja18 NKT lymphocyte antigen caused by beta-D-glucosylceramide synthase deficiency
Abstract
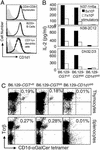
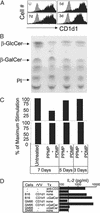
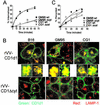
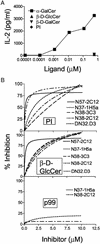
Va14Ja18 natural T (NKT) cells play an immunoregulatory role, which is controlled by a self glycolipid(s) presented by CD1d. Although the synthetic antigen alpha-D-galactosylceramide (alpha-D-GalCer) stimulates all Va14Ja18 NKT cells, alpha-anomeric D-glycosylceramides are currently unknown in mammals. We have used beta-D-GalCer-deficient mice and beta-D-glucosylceramide (beta-D-GlcCer)-deficient cells to define the chemical nature of a natural NKT cell antigen. beta-D-GalCer-deficient mice exhibit normal NKT cell development and function, and cells from these animals potently stimulate NKT hybridomas. In striking contrast, the same hybridomas fail to react to CD1d1 expressed by a beta-D-GlcCer-deficient cell line. Importantly, human beta-D-GlcCer synthase cDNA transfer, and hence the biosynthesis of beta-D-GlcCer, restores the recognition of mutant cells expressing CD1d1 by the Va14Ja18 NKT hybridomas. Additionally, suppression of beta-D-GlcCer synthesis inhibits antigen presentation to Va14Ja18 NKT cells. The possibility that beta-D-GlcCer itself is the natural NKT cell antigen was excluded because it was unable to activate NKT hybridomas in a cell-free antigen-presentation assay. These findings suggest that beta-D-GlcCer may play an important role in generating and/or loading a natural Va14Ja18 NKT antigen.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

