Evidence for the production of trioxygen species during antibody-catalyzed chemical modification of antigens
- PMID: 12576548
- PMCID: PMC149858
- DOI: 10.1073/pnas.0437831100
Evidence for the production of trioxygen species during antibody-catalyzed chemical modification of antigens
Abstract
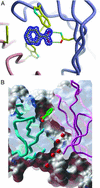
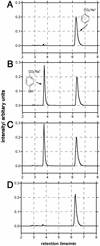
Recent work in our laboratory showed that products formed by the antibody-catalyzed water-oxidation pathway can kill bacteria. Dihydrogen peroxide, the end product of this pathway, was found to be necessary, but not sufficient, for the observed efficiency of bacterial killing. The search for further bactericidal agents that might be formed along the pathway led to the recognition of an oxidant that, in its interaction with chemical probes, showed the chemical signature of ozone. Here we report that the antibody-catalyzed water-oxidation process is capable of regioselectively converting antibody-bound benzoic acid into para-hydroxy benzoic acid as well as regioselectively hydroxylating the 4-position of the phenyl ring of a single tryptophan residue located in the antibody molecule. We view the occurrence of these highly selective chemical reactions as evidence for the formation of a short-lived hydroxylating radical species within the antibody molecule. In line with our previously presented hypothesis according to which the singlet-oxygen ((1)O*(2)) induced antibody-catalyzed water-oxidation pathways proceeds via the formation of dihydrogen trioxide (H(2)O(3)), we now consider the possibility that the hydroxylating species might be the hydrotrioxy radical HO(3)*, and we point to the remarkable potential of this either H(2)O(3)- or O(3)-derivable species to act as a masked hydroxyl radical HO* in a biological environment.
Figures


References
-
- Wentworth P, Jr, Jones L H, Wentworth A D, Zhu X, Larsen N A, Wilson I A, Xu X, Goddard W A, III, Janda K D, Eschenmoser A, Lerner R A. Science. 2001;293:1806–1809. - PubMed
-
- Wentworth P, Jr, McDunn J, Wentworth A D, Takeuchi C, Nieva J, Janda K D, Eschenmoser A, Lerner R A. Science. 2002;298:2195–2199. - PubMed
-
- Weiss J. Trans Faraday Soc. 1935;31:668–681.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

