Substrate-specific function of the translocon-associated protein complex during translocation across the ER membrane
- PMID: 12578908
- PMCID: PMC2173754
- DOI: 10.1083/jcb.200210095
Substrate-specific function of the translocon-associated protein complex during translocation across the ER membrane
Abstract
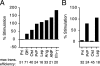
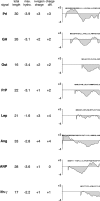
Although the transport of model proteins across the mammalian ER can be reconstituted with purified Sec61p complex, TRAM, and signal recognition particle receptor, some substrates, such as the prion protein (PrP), are inefficiently or improperly translocated using only these components. Here, we purify a factor needed for proper translocation of PrP and identify it as the translocon-associated protein (TRAP) complex. Surprisingly, TRAP also stimulates vectorial transport of many, but not all, other substrates in a manner influenced by their signal sequences. Comparative analyses of several natural signal sequences suggest that a dependence on TRAP for translocation is not due to any single physical parameter, such as hydrophobicity of the signal sequence. Instead, a functional property of the signal, efficiency of its post-targeting role in initiating substrate translocation, correlates inversely with TRAP dependence. Thus, maximal translocation independent of TRAP can only be achieved with a signal sequence, such as the one from prolactin, whose strong interaction with the translocon mediates translocon gating shortly after targeting. These results identify the TRAP complex as a functional component of the translocon and demonstrate that it acts in a substrate-specific manner to facilitate the initiation of protein translocation.
Figures






References
-
- Andrews, D.W., and A.E. Johnson. 1996. The translocon: more than a hole in the ER membrane? Trends Biochem. Sci. 21:365–369. - PubMed
-
- Beckmann, R., D. Bubeck, R. Grassucci, P. Penczek, A. Verschoor, G. Blobel, and J. Frank. 1997. Alignment of conduits for the nascent polypeptide chain in the ribosome-Sec61 complex. Science. 278:2123–2126. - PubMed
-
- Crowley, K.S., S. Liao, V.E. Worrell, G.D. Reinhart, and A.E. Johnson. 1994. Secretory proteins move through the endoplasmic reticulum membrane via an aqueous, gated pore. Cell. 78:461–471. - PubMed
-
- Gorlich, D., and T.A. Rapoport. 1993. Protein translocation into proteoliposomes reconstituted from purified components of the endoplasmic reticulum membrane. Cell. 75:615–630. - PubMed
-
- Gorlich, D., E. Hartmann, S. Prehn, and T.A. Rapoport. 1992. a. A protein of the endoplasmic reticulum involved early in polypeptide translocation. Nature. 357:47–52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

