Loading of the human 9-1-1 checkpoint complex onto DNA by the checkpoint clamp loader hRad17-replication factor C complex in vitro
- PMID: 12578958
- PMCID: PMC149884
- DOI: 10.1073/pnas.0437927100
Loading of the human 9-1-1 checkpoint complex onto DNA by the checkpoint clamp loader hRad17-replication factor C complex in vitro
Abstract
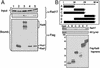
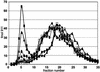
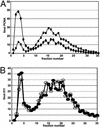
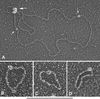
The human DNA damage sensors, Rad17-replication factor C (Rad17-RFC) and the Rad9-Rad1-Hus1 (9-1-1) checkpoint complex, are thought to be involved in the early steps of the DNA damage checkpoint response. Rad17-RFC and the 9-1-1 complex have been shown to be structurally similar to the replication factors, RFC clamp loader and proliferating cell nuclear antigen polymerase clamp, respectively. Here, we demonstrate functional similarities between the replication and checkpoint clamp loader/DNA clamp pairs. When all eight subunits of the two checkpoint complexes are coexpressed in insect cells, a stable Rad17-RFC/9-1-1 checkpoint supercomplex forms in vivo and is readily purified. The two individually purified checkpoint complexes also form a supercomplex in vitro, which depends on ATP and is mediated by interactions between Rad17 and Rad9. Rad17-RFC binds to nicked circular, gapped, and primed DNA and recruits the 9-1-1 complex in an ATP-dependent manner. Electron microscopic analyses of the reaction products indicate that the 9-1-1 ring is clamped around the DNA.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

