Loss of WAVE-1 causes sensorimotor retardation and reduced learning and memory in mice
- PMID: 12578964
- PMCID: PMC149900
- DOI: 10.1073/pnas.0438033100
Loss of WAVE-1 causes sensorimotor retardation and reduced learning and memory in mice
Abstract
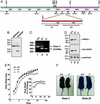
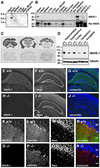
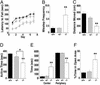
The Scar/WAVE family of scaffolding proteins organize molecular networks that relay signals from the GTPase Rac to the actin cytoskeleton. The WAVE-1 isoform is a brain-specific protein expressed in variety of areas including the regions of the hippocampus and the Purkinje cells of the cerebellum. Targeted disruption of the WAVE-1 gene generated mice with reduced anxiety, sensorimotor retardation, and deficits in hippocampal-dependent learning and memory. These sensorimotor and cognitive deficits are analogous to the symptoms of patients with 3p-syndrome mental retardation who are haploinsufficient for WRP/MEGAP, a component of the WAVE-1 signaling network. Thus WAVE-1 is required for normal neural functioning.
Figures
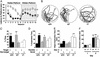
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

