Visualization of synaptotagmin I oligomers assembled onto lipid monolayers
- PMID: 12578982
- PMCID: PMC149962
- DOI: 10.1073/pnas.0435872100
Visualization of synaptotagmin I oligomers assembled onto lipid monolayers
Abstract
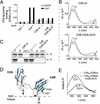
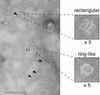
Neuronal exocytosis is mediated by Ca(2+)-triggered rearrangements between proteins and lipids that result in the opening and dilation of fusion pores. Synaptotagmin I (syt I) is a Ca(2+)-sensing protein proposed to regulate fusion pore dynamics via Ca(2+)-promoted binding of its cytoplasmic domain (C2A-C2B) to effector molecules, including anionic phospholipids and other copies of syt. Functional studies indicate that Ca(2+)-triggered oligomerization of syt is a critical step in excitation-secretion coupling; however, this activity has recently been called into question. Here, we show that Ca(2+) does not drive the oligomerization of C2A-C2B in solution. However, analysis of Ca(2+).C2A-C2B bound to lipid monolayers, using electron microscopy, revealed the formation of ring-like heptameric oligomers that are approximately 11 nm long and approximately 11 nm in diameter. In some cases, C2A-C2B also assembled into long filaments. Oligomerization, but not membrane binding, was disrupted by neutralization of two lysine residues (K326,327) within the C2B domain of syt. These data indicate that Ca(2+) first drives C2A-C2B.membrane interactions, resulting in conformational changes that trigger a subsequent C2B-mediated oligomerization step. Ca(2+)-mediated rearrangements between syt subunits may regulate the opening or dilation kinetics of fusion pores or may play a role in endocytosis after fusion.
Figures

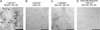

Similar articles
-
Mechanism of the calcium-dependent multimerization of synaptotagmin VII mediated by its first and second C2 domains.J Biol Chem. 2001 Jul 20;276(29):27670-6. doi: 10.1074/jbc.M100851200. Epub 2001 May 23. J Biol Chem. 2001. PMID: 11373279
-
The C2B domain of synaptotagmin is a Ca(2+)-sensing module essential for exocytosis.J Cell Biol. 2000 Sep 4;150(5):1125-36. doi: 10.1083/jcb.150.5.1125. J Cell Biol. 2000. PMID: 10974000 Free PMC article.
-
C2A activates a cryptic Ca(2+)-triggered membrane penetration activity within the C2B domain of synaptotagmin I.Proc Natl Acad Sci U S A. 2002 Feb 5;99(3):1665-70. doi: 10.1073/pnas.032541099. Epub 2002 Jan 22. Proc Natl Acad Sci U S A. 2002. PMID: 11805296 Free PMC article.
-
The C2 domains of synaptotagmin--partners in exocytosis.Trends Biochem Sci. 2004 Mar;29(3):143-51. doi: 10.1016/j.tibs.2004.01.008. Trends Biochem Sci. 2004. PMID: 15003272 Review.
-
Role of synaptotagmin in Ca2+-triggered exocytosis.Biochem J. 2002 Aug 15;366(Pt 1):1-13. doi: 10.1042/BJ20020776. Biochem J. 2002. PMID: 12047220 Free PMC article. Review.
Cited by
-
A synaptotagmin suppressor screen indicates SNARE binding controls the timing and Ca2+ cooperativity of vesicle fusion.Elife. 2017 Sep 12;6:e28409. doi: 10.7554/eLife.28409. Elife. 2017. PMID: 28895532 Free PMC article.
-
Synaptotagmin-mediated bending of the target membrane is a critical step in Ca(2+)-regulated fusion.Cell. 2009 Aug 21;138(4):709-21. doi: 10.1016/j.cell.2009.05.049. Cell. 2009. PMID: 19703397 Free PMC article.
-
C2B polylysine motif of synaptotagmin facilitates a Ca2+-independent stage of synaptic vesicle priming in vivo.Mol Biol Cell. 2006 Dec;17(12):5211-26. doi: 10.1091/mbc.e06-07-0622. Epub 2006 Sep 20. Mol Biol Cell. 2006. PMID: 16987956 Free PMC article.
-
Prevalent mechanism of membrane bridging by synaptotagmin-1.Proc Natl Acad Sci U S A. 2013 Aug 20;110(34):E3243-52. doi: 10.1073/pnas.1310327110. Epub 2013 Aug 5. Proc Natl Acad Sci U S A. 2013. PMID: 23918375 Free PMC article.
-
Involvement of Ca2+ channel synprint site in synaptic vesicle endocytosis.J Neurosci. 2010 Jan 13;30(2):655-60. doi: 10.1523/JNEUROSCI.3214-09.2010. J Neurosci. 2010. PMID: 20071530 Free PMC article.
References
-
- Katz B. The Release of Neural Transmitter Substances. Springfield, IL: Thomas; 1969.
-
- Lindau M, Almers W. Curr Opin Cell Biol. 1995;7:509–517. - PubMed
-
- Augustine G J. Curr Opin Neurobiol. 2001;11:320–326. - PubMed
-
- Takei K, Haucke V. Trends Cell Biol. 2001;11:385–391. - PubMed
-
- Chapman E R. Nat Rev Mol Cell Biol. 2002;3:498–508. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

