Hypersusceptibility of cystic fibrosis mice to chronic Pseudomonas aeruginosa oropharyngeal colonization and lung infection
- PMID: 12578988
- PMCID: PMC149939
- DOI: 10.1073/pnas.0437901100
Hypersusceptibility of cystic fibrosis mice to chronic Pseudomonas aeruginosa oropharyngeal colonization and lung infection
Abstract
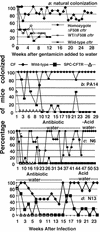
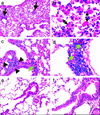
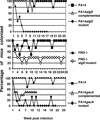
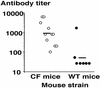
No transgenic cystic fibrosis (CF) mouse model developed to date mimics the major clinical phenotype found in humans with CF, chronic Pseudomonas aeruginosa lung infection. In a transgenic CF transmembrane conductance regulator (cftr) mouse colony, we found WT, heterozygous, and homozygous CF mice housed in the same cage became chronically colonized in the oropharynx with environmental P. aeruginosa when the bacterium was present in drinking water. Elimination of P. aeruginosa from drinking water resulted in clearance in most WT and CF heterozygous, but not homozygous mice. For experimental evaluation, a combination of specific animal husbandry techniques and an oral infection route showed cftr(-/-) mice but not WT mice can be chronically colonized by P. aeruginosa with subsequent lung translocation, yielding a pathologic picture indicative of chronic lung infection. In some instances, mucoid isolates of P. aeruginosa were recovered from lungs, indicating conditions were present for conversion to mucoidy. Overexpression of human CFTR in the lungs of WT mice markedly accelerated the clearance rate of P. aeruginosa, demonstrating that lung levels of CFTR play an important role in defense against infection. P. aeruginosa mutants unable to express the surface polysaccharide alginate or the global regulator GacA were deficient in their ability to colonize the mice. CF mice made potent immune responses to P. aeruginosa outer membrane antigens. Overall, we found that under the proper conditions, transgenic CF mice are hypersusceptible to P. aeruginosa colonization and infection and can be used for evaluations of lung pathophysiology, bacterial virulence, and development of therapies aimed at treating CF lung disease.
Figures

References
-
- Grubb B R, Boucher R C. Physiol Rev. 1999;79:S193–S214. - PubMed
-
- Davidson D J, Dorin J R, Mclachlan G, Ranaldi V, Lamb D, Doherty C, Govan J, Porteous D J. Nat Genet. 1995;9:351–357. - PubMed
-
- Colledge W H, Abella B S, Southern K W, Ratcliff R, Jiang C W, Cheng S H, Macvinish L J, Anderson J R, Cuthbert A W, Evans M J. Nat Genet. 1995;10:445–452. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

