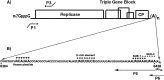
Cis-acting regulatory elements in the potato virus X 3' non-translated region differentially affect minus-strand and plus-strand RNA accumulation
- PMID: 12581634
- PMCID: PMC7126608
- DOI: 10.1016/s0022-2836(02)01369-4
Cis-acting regulatory elements in the potato virus X 3' non-translated region differentially affect minus-strand and plus-strand RNA accumulation
Abstract
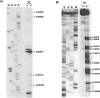
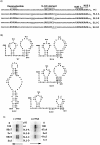
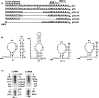
The 72nt 3' non-translated region (NTR) of potato virus X (PVX) RNA is identical in all sequenced PVX strains and contains sequences that are conserved among all potexviruses. Computer folding of the 3' NTR sequence predicted three stem-loop structures (SL1, SL2, and SL3 in the 3' to 5' direction), which generally were supported by solution structure analyses. The importance of these sequence and/or structural elements to PVX RNA accumulation was further analyzed by inoculation of Nicotiana tabacum (NT-1) protoplasts with PVX transcripts containing mutations in the 3' NTR. Analyses of RNA accumulation by S(1) nuclease protection indicated that multiple sequence elements throughout the 3' NTR were important for minus-strand RNA accumulation. Formation of SL3 was required for accumulation of minus-strand RNA, whereas SL1 and SL2 formation were less important. However, sequences within all of these predicted structures were required for minus-strand RNA accumulation, including a conserved hexanucleotide sequence element in the loop of SL3, and the CU nucleotide in a U-rich sequence within SL2. In contrast, 13 nucleotides that were predicted to reside in SL1 could be deleted without any significant reduction in minus or plus-strand RNA levels. Potential polyadenylation signals (near upstream elements; NUEs) in the 3' NTR of PVX RNA were more important for plus-strand RNA accumulation than for minus-strand RNA accumulation. In addition, one of these NUEs overlapped with other sequence required for optimal minus-strand RNA levels. These data indicate that the PVX 3' NTR contains multiple, overlapping elements that influence accumulation of both minus and plus-strand RNA.
Figures

 ), moderate (
), moderate ( ), or strong (
), or strong ( ). The intensities of uridine modification by CMCT are denoted as low (
). The intensities of uridine modification by CMCT are denoted as low ( ), moderate (
), moderate ( ), or strong (
), or strong ( ). Filled circles represent ambiguous CMCT reactivity.
). Filled circles represent ambiguous CMCT reactivity.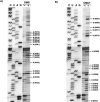




Similar articles
-
Long-distance RNA-RNA interactions between terminal elements and the same subset of internal elements on the potato virus X genome mediate minus- and plus-strand RNA synthesis.RNA. 2007 Feb;13(2):267-80. doi: 10.1261/rna.243607. Epub 2006 Dec 21. RNA. 2007. PMID: 17185361 Free PMC article.
-
Stem-loop structure in the 5' region of potato virus X genome required for plus-strand RNA accumulation.J Mol Biol. 1998 Dec 4;284(3):591-608. doi: 10.1006/jmbi.1998.2174. J Mol Biol. 1998. PMID: 9826501
-
Mutations that alter a repeated ACCA element located at the 5' end of the Potato virus X genome affect RNA accumulation.Virology. 2008 Aug 15;378(1):133-41. doi: 10.1016/j.virol.2008.05.004. Epub 2008 Jun 26. Virology. 2008. PMID: 18589472
-
Molecular biology of potexviruses: recent advances.J Gen Virol. 2007 Jun;88(Pt 6):1643-1655. doi: 10.1099/vir.0.82667-0. J Gen Virol. 2007. PMID: 17485523 Review.
-
Cis-acting RNA elements in human and animal plus-strand RNA viruses.Biochim Biophys Acta. 2009 Sep-Oct;1789(9-10):495-517. doi: 10.1016/j.bbagrm.2009.09.007. Epub 2009 Sep 23. Biochim Biophys Acta. 2009. PMID: 19781674 Free PMC article. Review.
Cited by
-
Hsp90 interacts specifically with viral RNA and differentially regulates replication initiation of Bamboo mosaic virus and associated satellite RNA.PLoS Pathog. 2012;8(5):e1002726. doi: 10.1371/journal.ppat.1002726. Epub 2012 May 24. PLoS Pathog. 2012. PMID: 22654666 Free PMC article.
-
Intragenomic Long-Distance RNA-RNA Interactions in Plus-Strand RNA Plant Viruses.Front Microbiol. 2018 Apr 4;9:529. doi: 10.3389/fmicb.2018.00529. eCollection 2018. Front Microbiol. 2018. PMID: 29670583 Free PMC article. Review.
-
Live-cell RNA imaging with the inactivated endonuclease Csy4 enables new insights into plant virus transport through plasmodesmata.PLoS Pathog. 2025 Apr 9;21(4):e1013049. doi: 10.1371/journal.ppat.1013049. eCollection 2025 Apr. PLoS Pathog. 2025. PMID: 40203052 Free PMC article.
-
Maintaining the structural integrity of the Bamboo mosaic virus 3' untranslated region is necessary for retaining the catalytic constant for minus-strand RNA synthesis.Virol J. 2013 Jun 24;10:208. doi: 10.1186/1743-422X-10-208. Virol J. 2013. PMID: 23800142 Free PMC article.
-
Evidence of pervasive biologically functional secondary structures within the genomes of eukaryotic single-stranded DNA viruses.J Virol. 2014 Feb;88(4):1972-89. doi: 10.1128/JVI.03031-13. Epub 2013 Nov 27. J Virol. 2014. PMID: 24284329 Free PMC article.
References
-
- Dreher T.W. Functions of the 3′-untranslated regions of positive strand RNA viral genomes. Annu. Rev. Phytopathol. 1999;37:151–174. - PubMed
-
- Hemenway C.L., Lommel S.A. Manipulating plant viral RNA transcription signals. Genet. Eng. (NY) 2000;22:171–195. - PubMed
-
- Klovins J., van Duin J. A long-range pseudoknot in Qβ RNA is essential for replication. J. Mol. Biol. 1999;294:875–884. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

