Cdc24, the GDP-GTP exchange factor for Cdc42, is required for invasive hyphal growth of Candida albicans
- PMID: 12582118
- PMCID: PMC141177
- DOI: 10.1128/EC.2.1.9-18.2003
Cdc24, the GDP-GTP exchange factor for Cdc42, is required for invasive hyphal growth of Candida albicans
Abstract
Candida albicans, the most common human fungal pathogen, is particularly problematic for immunocompromised individuals. The reversible transition of this fungal pathogen to a filamentous form that invades host tissue is important for its virulence. Although different signaling pathways such as a mitogen-activated protein kinase and a protein kinase A cascade are critical for this morphological transition, the function of polarity establishment proteins in this process has not been determined. We examined the role of four different polarity establishment proteins in C. albicans invasive growth and virulence by using strains in which one copy of each gene was deleted and the other copy expressed behind the regulatable promoter MET3. Strikingly, mutants with ectopic expression of either the Rho G-protein Cdc42 or its exchange factor Cdc24 are unable to form invasive hyphal filaments and germ tubes in response to serum or elevated temperature and yet grow normally as a budding yeast. Furthermore, these mutants are avirulent in a mouse model for systemic infection. This function of the Cdc42 GTPase module is not simply a general feature of polarity establishment proteins. Mutants with ectopic expression of the SH3 domain containing protein Bem1 or the Ras-like G-protein Bud1 can grow in an invasive fashion and are virulent in mice, albeit with reduced efficiency. These results indicate that a specific regulation of Cdc24/Cdc42 activity is required for invasive hyphal growth and suggest that these proteins are required for pathogenicity of C. albicans.
Figures





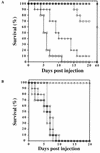
Similar articles
-
Regulation of the Cdc42/Cdc24 GTPase module during Candida albicans hyphal growth.Eukaryot Cell. 2005 Mar;4(3):588-603. doi: 10.1128/EC.4.3.588-603.2005. Eukaryot Cell. 2005. PMID: 15755921 Free PMC article.
-
Rsr1 focuses Cdc42 activity at hyphal tips and promotes maintenance of hyphal development in Candida albicans.Eukaryot Cell. 2013 Apr;12(4):482-95. doi: 10.1128/EC.00294-12. Epub 2012 Dec 7. Eukaryot Cell. 2013. PMID: 23223038 Free PMC article.
-
Control of the yeast bud-site assembly GTPase Cdc42. Catalysis of guanine nucleotide exchange by Cdc24 and stimulation of GTPase activity by Bem3.J Biol Chem. 1994 Jan 28;269(4):2369-72. J Biol Chem. 1994. PMID: 8300560
-
Ras-related GTPases and the cytoskeleton.Mol Biol Cell. 1992 May;3(5):475-9. doi: 10.1091/mbc.3.5.475. Mol Biol Cell. 1992. PMID: 1611153 Free PMC article. Review.
-
Messenger RNA transport in the opportunistic fungal pathogen Candida albicans.Curr Genet. 2017 Dec;63(6):989-995. doi: 10.1007/s00294-017-0707-6. Epub 2017 May 16. Curr Genet. 2017. PMID: 28512683 Free PMC article. Review.
Cited by
-
Targeting dermatophyte Cdc42 and Rac GTPase signaling to hinder hyphal elongation and virulence.iScience. 2024 May 28;27(6):110139. doi: 10.1016/j.isci.2024.110139. eCollection 2024 Jun 21. iScience. 2024. PMID: 38952678 Free PMC article.
-
Effects of low-shear modeled microgravity on cell function, gene expression, and phenotype in Saccharomyces cerevisiae.Appl Environ Microbiol. 2006 Jul;72(7):4569-75. doi: 10.1128/AEM.03050-05. Appl Environ Microbiol. 2006. PMID: 16820445 Free PMC article.
-
Regulatory circuitry governing fungal development, drug resistance, and disease.Microbiol Mol Biol Rev. 2011 Jun;75(2):213-67. doi: 10.1128/MMBR.00045-10. Microbiol Mol Biol Rev. 2011. PMID: 21646428 Free PMC article. Review.
-
Regulation of the Cdc42/Cdc24 GTPase module during Candida albicans hyphal growth.Eukaryot Cell. 2005 Mar;4(3):588-603. doi: 10.1128/EC.4.3.588-603.2005. Eukaryot Cell. 2005. PMID: 15755921 Free PMC article.
-
Polarized hyphal growth in Candida albicans requires the Wiskott-Aldrich Syndrome protein homolog Wal1p.Eukaryot Cell. 2004 Apr;3(2):471-82. doi: 10.1128/EC.3.2.471-482.2004. Eukaryot Cell. 2004. PMID: 15075276 Free PMC article.
References
-
- Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. - PubMed
-
- Bose, I., J. E. Irazoqui, J. J. Moskow, E. S. Bardes, T. R. Zyla, and D. J. Lew. 2001. Assembly of scaffold-mediated complexes containing Cdc42p, the exchange factor Cdc24p, and the effector Cla4p required for cell cycle-regulated phosphorylation of Cdc24p. J. Biol. Chem. 276:7176-7186. - PubMed
-
- Brown, A. J., and N. A. Gow. 1999. Regulatory networks controlling Candida albicans morphogenesis. Trends Microbiol. 7:333-338. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

