Cell biology of mating in Candida albicans
- PMID: 12582122
- PMCID: PMC141171
- DOI: 10.1128/EC.2.1.49-61.2003
Cell biology of mating in Candida albicans
Abstract
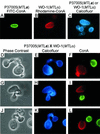
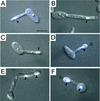
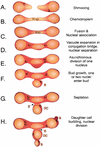
It was recently demonstrated that strains homozygous for either of the mating type-like loci MTLa and MTLalpha of Candida albicans undergo white-opaque switching and that expression of the opaque-phase phenotype greatly enhances mating between strains. Exploiting the latter property to obtain high-frequency mating, we have characterized the cell biology of the mating process of C. albicans. Employing continuous videomicroscopy, computer-assisted three-dimensional reconstruction of living cells, and fluorescence microscopy, we have monitored the mating-associated processes of conjugation, tube formation, fusion, budding, septum formation, and daughter cell development and the spatial and temporal dynamics of nuclear migration and division. From these observations, a model for the stages in C. albicans mating is formulated. The stages include shmooing, chemotropism of conjugation tubes, fusion of tubes and nuclear association, vacuole expansion and nuclear separation in the conjugation bridge, asynchronous nuclear division in the zygote, bud growth, nuclear migration into the daughter cell, septation, and daughter cell budding. Since there was no cytological indication of karyogamy, genetic experiments were performed to assess marker segregation. Recombination was not observed, suggesting that mating takes place in the absence of karyogamy between naturally occurring, homozygous a and alpha strains. This study provides the first description of the cell biology of the mating process of C. albicans.
Figures







References
-
- Byers, B. 1981. Cytology of the yeast life cycle, p. 59. In J. N. Strethern (ed.), The molecular biology of the yeast Saccharomyces: life cycle and inheritance. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

