Constitutively active protein kinase A disrupts motility and chemotaxis in Dictyostelium discoideum
- PMID: 12582123
- PMCID: PMC141174
- DOI: 10.1128/EC.2.1.62-75.2003
Constitutively active protein kinase A disrupts motility and chemotaxis in Dictyostelium discoideum
Abstract
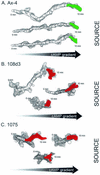
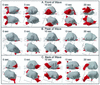
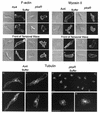
The deletion of the gene for the regulatory subunit of protein kinase A (PKA) results in constitutively active PKA in the pkaR mutant. To investigate the role of PKA in the basic motile behavior and chemotaxis of Dictyostelium discoideum, pkaR mutant cells were subjected to computer-assisted two- and three-dimensional motion analysis. pkaR mutant cells crawled at only half the speed of wild-type cells in buffer, chemotaxed in spatial gradients of cyclic AMP (cAMP) but with reduced efficiency, were incapable of suppressing lateral pseudopods in the front of temporal waves of cAMP, a requirement for natural chemotaxis, did not exhibit the normal velocity surge in response to the front of a wave, and were incapable of chemotaxing toward an aggregation center in natural waves generated by wild-type cells that made up the majority of cells in mixed cultures. Many of the behavioral defects appeared to be the result of the constitutively ovoid shape of the pkaR mutant cells, which forced the dominant pseudopod off the substratum and to the top of the cell body. The behavioral abnormalities that pkaR mutant cells shared with regA mutant cells are discussed by considering the pathway ERK2 perpendicular RegA perpendicular [cAMP] --> PKA, which emanates from the front of a wave. The results demonstrate that cells must suppress PKA activity in order to elongate along a substratum, suppress lateral-pseudopod formation, and crawl and chemotax efficiently. The results also implicate PKA activation in dismantling cell polarity at the peak and in the back of a natural cAMP wave.
Figures







Similar articles
-
Intracellular role of adenylyl cyclase in regulation of lateral pseudopod formation during Dictyostelium chemotaxis.Eukaryot Cell. 2005 Apr;4(4):775-86. doi: 10.1128/EC.4.4.775-786.2005. Eukaryot Cell. 2005. PMID: 15821137 Free PMC article.
-
A contextual framework for characterizing motility and chemotaxis mutants in Dictyostelium discoideum.J Muscle Res Cell Motil. 2002;23(7-8):659-72. doi: 10.1023/a:1024459124427. J Muscle Res Cell Motil. 2002. PMID: 12952065 Review.
-
The internal phosphodiesterase RegA is essential for the suppression of lateral pseudopods during Dictyostelium chemotaxis.Mol Biol Cell. 2000 Aug;11(8):2803-20. doi: 10.1091/mbc.11.8.2803. Mol Biol Cell. 2000. PMID: 10930471 Free PMC article.
-
Regulated protein degradation controls PKA function and cell-type differentiation in Dictyostelium.Genes Dev. 2001 Jun 1;15(11):1435-48. doi: 10.1101/gad.871101. Genes Dev. 2001. PMID: 11390363 Free PMC article.
-
Role of PKA in the timing of developmental events in Dictyostelium cells.Microbiol Mol Biol Rev. 1998 Sep;62(3):684-94. doi: 10.1128/MMBR.62.3.684-694.1998. Microbiol Mol Biol Rev. 1998. PMID: 9729606 Free PMC article. Review.
Cited by
-
Eukaryotic chemotaxis.Wiley Interdiscip Rev Syst Biol Med. 2009 Jul-Aug;1(1):141-149. doi: 10.1002/wsbm.28. Wiley Interdiscip Rev Syst Biol Med. 2009. PMID: 20648241 Free PMC article.
-
Intracellular role of adenylyl cyclase in regulation of lateral pseudopod formation during Dictyostelium chemotaxis.Eukaryot Cell. 2005 Apr;4(4):775-86. doi: 10.1128/EC.4.4.775-786.2005. Eukaryot Cell. 2005. PMID: 15821137 Free PMC article.
-
A contextual framework for characterizing motility and chemotaxis mutants in Dictyostelium discoideum.J Muscle Res Cell Motil. 2002;23(7-8):659-72. doi: 10.1023/a:1024459124427. J Muscle Res Cell Motil. 2002. PMID: 12952065 Review.
-
Protein kinase A regulates the Ras, Rap1 and TORC2 pathways in response to the chemoattractant cAMP in Dictyostelium.J Cell Sci. 2017 May 1;130(9):1545-1558. doi: 10.1242/jcs.177170. Epub 2017 Mar 16. J Cell Sci. 2017. PMID: 28302905 Free PMC article.
-
Imaging of cell migration.EMBO J. 2006 Aug 9;25(15):3480-93. doi: 10.1038/sj.emboj.7601227. EMBO J. 2006. PMID: 16900100 Free PMC article. Review.
References
-
- Alcantara, F., and M. Monk. 1974. Signal propagation during aggregation in the slime mould Dictyostelium discoideum. J. Gen. Microbiol. 85:321-324. - PubMed
-
- Anjard, C., R. Pinaud, R. Kay, and C. D. Reymond. 1992. Overexpression of DdPK2 protein kinase causes rapid development and affects the intra-cellular cAMP pathway of Dictyostelium discoideum. Development 115:785-790. - PubMed
-
- Anjard, C., M. van Bemmelen, M. Veron, and C. D. Reymond. 1997. SDF, a new spore differentiation factor secreted by Dictyostelium cells, is phosphorylated by the cAMP dependent protein kinase. Differentiation 62:43-49. - PubMed
-
- Aubry, L., and R. A. Firtel. 1999. Integration of signaling networks that regulate Dictyostelium differentiation. Annu. Rev. Cell Dev. Biol. 15:469-517. - PubMed
-
- Aubry, L., M. Maeda, R. Insall, P. N. Devreotes, and R. A. Firtel. 1997. The Dictyostelium mitogen-activated protein kinase ERK2 is regulated by ras and cAMP-dependent protein kinase (PKA) and mediates PKA function. J. Biol. Chem. 272:3883-3886. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

