Characterization of the human beta-globin downstream promoter region
- PMID: 12582249
- PMCID: PMC150227
- DOI: 10.1093/nar/gkg209
Characterization of the human beta-globin downstream promoter region
Abstract
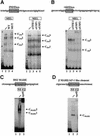
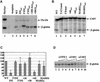
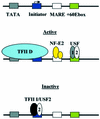
The human beta-globin gene is abundantly expressed specifically in adult erythroid cells. Stage-specific transcription is regulated principally by promoter proximal cis-regulatory elements. The basal promoter contains a non-canonical TATA-like motif as well as an initiator element. These two elements have been shown to interact with the TFII-D complex. Here we show that in addition to the TATA and initiator elements, conserved E-box motifs are located in the beta-globin downstream promoter. One of the E-box motifs overlaps the initiator and this composite element interacts with USF1 and TFII-I in vitro. Another E-box, located 60 bp 3' to the transcription initiation site, interacts with USF1 and USF2. Mutations of either the initiator or the downstream E-box impair transcription of the beta-globin gene in vitro. Mutations of a putative NF-E2-binding site in the downstream promoter region do not affect transcription in vitro. USF1, USF2, TFII-I and p45 can be crosslinked to a beta-globin promoter fragment in MEL cells in vivo, whereas only TFII-I and USF2 crosslink to the beta-globin gene in K562 cells. The summary data demonstrate that in addition to the well-characterized interactions of the TFII-D complex with the basal promoter, E-box motifs contribute to the efficient formation of transcription complexes on the adult beta-globin gene.
Figures



Similar articles
-
Transcriptional activation of human zeta 2 globin promoter by the alpha globin regulatory element (HS-40): functional role of specific nuclear factor-DNA complexes.Mol Cell Biol. 1993 Apr;13(4):2298-308. doi: 10.1128/mcb.13.4.2298-2308.1993. Mol Cell Biol. 1993. PMID: 8455611 Free PMC article.
-
Genomic footprinting and sequencing of human beta-globin locus. Tissue specificity and cell line artifact.J Biol Chem. 1994 Mar 18;269(11):8287-95. J Biol Chem. 1994. PMID: 8132552
-
Activation of the delta-globin gene by the beta-globin gene CACCC motif.Blood Cells Mol Dis. 1999 Jun-Aug;25(3-4):193-209. doi: 10.1006/bcmd.1999.0245. Blood Cells Mol Dis. 1999. PMID: 10575545
-
ChIPs of the beta-globin locus: unraveling gene regulation within an active domain.Curr Opin Genet Dev. 2002 Apr;12(2):170-7. doi: 10.1016/s0959-437x(02)00283-6. Curr Opin Genet Dev. 2002. PMID: 11893490 Review.
-
The DPE, a conserved downstream core promoter element that is functionally analogous to the TATA box.Cold Spring Harb Symp Quant Biol. 1998;63:75-82. doi: 10.1101/sqb.1998.63.75. Cold Spring Harb Symp Quant Biol. 1998. PMID: 10384272 Review. No abstract available.
Cited by
-
TFII-I/Gtf2i and Erythro-Megakaryopoiesis.Front Physiol. 2020 Sep 25;11:590180. doi: 10.3389/fphys.2020.590180. eCollection 2020. Front Physiol. 2020. PMID: 33101065 Free PMC article.
-
Synergistic and additive properties of the beta-globin locus control region (LCR) revealed by 5'HS3 deletion mutations: implication for LCR chromatin architecture.Mol Cell Biol. 2005 Aug;25(16):7033-41. doi: 10.1128/MCB.25.16.7033-7041.2005. Mol Cell Biol. 2005. PMID: 16055715 Free PMC article.
-
Distinct modes of gene regulation by a cell-specific transcriptional activator.Proc Natl Acad Sci U S A. 2009 Mar 17;106(11):4213-8. doi: 10.1073/pnas.0808347106. Epub 2009 Feb 27. Proc Natl Acad Sci U S A. 2009. PMID: 19251649 Free PMC article.
-
USF and NF-E2 cooperate to regulate the recruitment and activity of RNA polymerase II in the beta-globin gene locus.J Biol Chem. 2010 May 21;285(21):15894-905. doi: 10.1074/jbc.M109.098376. Epub 2010 Mar 17. J Biol Chem. 2010. PMID: 20236933 Free PMC article.
-
Compact zinc finger base editors that edit mitochondrial or nuclear DNA in vitro and in vivo.Nat Commun. 2022 Nov 23;13(1):7204. doi: 10.1038/s41467-022-34784-7. Nat Commun. 2022. PMID: 36418298 Free PMC article.
References
-
- Bulger M. and Groudine,M. (1999) Looping versus linking: toward a model for long-distance gene activation. Genes Dev., 13, 2465–2477. - PubMed
-
- Higgs D. (1998) Do LCRs open chromatin domains? Cell, 95, 299–302. - PubMed
-
- Engel J.D. and Tanimoto,K. (2000) Looping, linking and chromatin activity: new insights into β-globin locus regulation. Cell, 100, 499–502. - PubMed
-
- Levings P.P. and Bungert,J. (2002) The human β-globin locus control region: a center of attraction. Eur. J. Biochem., 269, 1589–1599. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

