Rapid generation of inducible mouse mutants
- PMID: 12582257
- PMCID: PMC150244
- DOI: 10.1093/nar/gng012
Rapid generation of inducible mouse mutants
Abstract
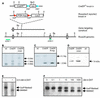
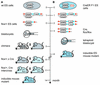
We have generated an optimized inducible recombination system for conditional gene targeting based on a Cre recombinase-steroid receptor fusion. This configuration allows efficient Cre-mediated recombination in most organs of the mouse upon induction, without detectable background activity. An ES cell line, was established that carries the inducible recombinase and a loxP-flanked lacZ reporter gene. Out of this line, completely ES cell-derived mice were efficiently produced through tetraploid blastocyst complementation, without the requirement of mouse breeding. Our findings provide a new concept allowing the generation of inducible mouse mutants within 6 months, as compared to 14 months using the current protocol.
Figures



Similar articles
-
Ligand-dependent genetic recombination in fibroblasts : a potentially powerful technique for investigating gene function in fibrosis.Am J Pathol. 2002 May;160(5):1609-17. doi: 10.1016/S0002-9440(10)61108-X. Am J Pathol. 2002. PMID: 12000713 Free PMC article.
-
Endothelium-specific Cre recombinase activity in flk-1-Cre transgenic mice.Dev Dyn. 2004 Feb;229(2):312-8. doi: 10.1002/dvdy.10416. Dev Dyn. 2004. PMID: 14745955
-
Conditional gene knockout using Cre recombinase.Mol Biotechnol. 2001 Mar;17(3):269-75. doi: 10.1385/MB:17:3:269. Mol Biotechnol. 2001. PMID: 11434315
-
Conditional mutagenesis in mice: the Cre/loxP recombination system.Int J Exp Pathol. 1996 Dec;77(6):269-78. Int J Exp Pathol. 1996. PMID: 9155661 Free PMC article. Review. No abstract available.
-
Beyond 'knock-out' mice: new perspectives for the programmed modification of the mammalian genome.Mol Hum Reprod. 1998 Oct;4(10):929-38. doi: 10.1093/molehr/4.10.929. Mol Hum Reprod. 1998. PMID: 9809673 Review.
Cited by
-
Conditional Deletion of Smad1 Ameliorates Glomerular Injury in Progressive Glomerulonephritis.Sci Rep. 2016 Aug 5;6:31216. doi: 10.1038/srep31216. Sci Rep. 2016. PMID: 27492138 Free PMC article.
-
Tetracycline-controlled transgene activation using the ROSA26-iM2-GFP knock-in mouse strain permits GFP monitoring of DOX-regulated transgene-expression.BMC Dev Biol. 2010 Sep 3;10:95. doi: 10.1186/1471-213X-10-95. BMC Dev Biol. 2010. PMID: 20815887 Free PMC article.
-
Mouse knock-out of IOP1 protein reveals its essential role in mammalian cytosolic iron-sulfur protein biogenesis.J Biol Chem. 2011 May 6;286(18):15797-805. doi: 10.1074/jbc.M110.201731. Epub 2011 Mar 2. J Biol Chem. 2011. PMID: 21367862 Free PMC article.
-
Relation between extent of myostatin depletion and muscle growth in mature mice.Am J Physiol Endocrinol Metab. 2009 Oct;297(4):E935-40. doi: 10.1152/ajpendo.00179.2009. Epub 2009 Aug 4. Am J Physiol Endocrinol Metab. 2009. PMID: 19654287 Free PMC article.
-
Control of inducible gene expression links cohesin to hematopoietic progenitor self-renewal and differentiation.Nat Immunol. 2018 Sep;19(9):932-941. doi: 10.1038/s41590-018-0184-1. Epub 2018 Aug 20. Nat Immunol. 2018. PMID: 30127433 Free PMC article.
References
-
- Lewandoski M. (2001) Conditional control of gene expression in the mouse. Nature Rev. Genet., 2, 743–755. - PubMed
-
- Kwan K.M. (2002) Conditional alleles in mice: practical considerations for tissue-specific knockouts. Genesis, 32, 49–62. - PubMed
-
- Gu H., Marth,J.D., Orban,P.C., Mossmann,H. and Rajewsky,K. (1994) Deletion of a DNA polymerase beta gene segment in T cells using cell type specific gene targeting. Science, 265, 103–106. - PubMed
-
- Kahn C.R., Bruning,J.C., Michael,M.D. and Kulkarni,R.N. (2000) Knockout mice challenge our concepts of glucose homeostasis and the pathogenesis of diabetes mellitus. J. Pediat. Endocrinol. Metab., 13, 1377–1383. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

