A rapid decrease in the expression of DMT1 and Dcytb but not Ireg1 or hephaestin explains the mucosal block phenomenon of iron absorption
- PMID: 12584213
- PMCID: PMC1773562
- DOI: 10.1136/gut.52.3.340
A rapid decrease in the expression of DMT1 and Dcytb but not Ireg1 or hephaestin explains the mucosal block phenomenon of iron absorption
Abstract
Background: A large oral dose of iron will reduce the absorption of a subsequent smaller dose of iron in a phenomenon known as mucosal block. Molecular analysis of this process may provide insights into the regulation of intestinal iron absorption.
Aims: To determine the effect of an oral bolus of iron on duodenal expression of molecules associated with intestinal iron transport in rats and to relate this to changes in iron absorption.
Methods: Rats were given an oral dose of iron and duodenal expression of divalent metal transporter 1 (DMT1), Dcytb, Ireg1, and hephaestin (Hp) was determined using the ribonuclease protection assay, western blotting, and immunofluorescence. Iron absorption was measured using radioactive (59)Fe.
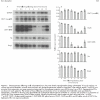
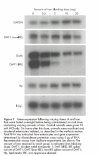
Results: A decrease in intestinal iron absorption occurred following an oral dose of iron and this was associated with increased enterocyte iron levels, as assessed by iron regulatory protein activity and immunoblotting for ferritin. Reduced absorption was also accompanied by a rapid decrease in expression of the mRNAs encoding the brush border iron transport molecules Dcytb and the iron responsive element (IRE) containing the splice variant of DMT1. No such change was seen in expression of the non-IRE splice variant of DMT1 or the basolateral iron transport molecules Ireg1 and Hp. Similar changes were observed at the protein level.
Conclusions: These data indicate that brush border, but not basolateral, iron transport components are regulated locally by enterocyte iron levels and support the hypothesis that systemic stimuli exert their primary effect on basolateral transport molecules.
Figures




References
-
- Conrad ME, Barton JC. Factors affecting iron balance. Am J Hematol 1981;10:199–225. - PubMed
-
- Crichton RR, Charloteaux-Wauters M. Iron storage and transport. Eur J Biochem 1987;164:485–506. - PubMed
-
- Bothwell TH, Pirzio-Biroli G, Finch CA. Iron absorption I. Factors influencing absorption. J Lab Clin Med 1958;51:24–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
