Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation
- PMID: 12584232
- PMCID: PMC1773546
- DOI: 10.1136/gut.52.3.439
Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation
Abstract
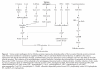
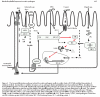
The effects of pathogenic organisms on host intestinal epithelial cells are vast. Innumerable signalling pathways are triggered leading ultimately to drastic changes in physiological functions. Here, the ways in which enteric bacterial pathogens utilise and impact on the three major physiological functions of the intestinal epithelium are discussed: alterations in the structure and function of the tight junction barrier, induction of fluid and electrolyte secretion, and activation of the inflammatory cascade. This field of investigation, which was virtually non-existent a decade ago, has now exploded, thus rapidly expanding our understanding of bacterial pathogenesis. Through increased delineation of the ways in which microbes alter host physiology, we simultaneous gain insight into the normal regulatory mechanisms of the intestinal epithelium.
Figures

References
-
- Ottingler MSL. Clostridium difficile toxin B induces reorganization of actin, vinculin, and talin in cultured cells. Exp Cell Res 1988;174:215–29. - PubMed
-
- Hecht G, Koutsouris A, Pothoulakis C, et al. Clostridium difficile toxin B disrupts the barrier function of T84 monolayers. Gastroenterology 1992;102:416–23. - PubMed
-
- Just I, Fritz G, Aktories K, et al. Clostridium difficile toxin B acts on the GTP-binding protein Rho. J Biol Chem 1994;269:10706–12. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
