Role of virus receptor Hyal2 in oncogenic transformation of rodent fibroblasts by sheep betaretrovirus env proteins
- PMID: 12584308
- PMCID: PMC149765
- DOI: 10.1128/jvi.77.5.2850-2858.2003
Role of virus receptor Hyal2 in oncogenic transformation of rodent fibroblasts by sheep betaretrovirus env proteins
Abstract
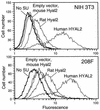
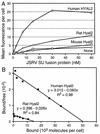
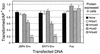
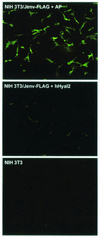
The ovine betaretroviruses jaagsiekte sheep retrovirus (JSRV) and enzootic nasal tumor virus (ENTV) cause contagious cancers in the lungs and upper airways of sheep and goats. Oncogenic transformation assays using mouse and rat fibroblasts have localized the transforming activity to the Env proteins encoded by these viruses, which require the putative lung and breast cancer tumor suppressor hyaluronidase 2 (Hyal2) to promote virus entry into cells. These results suggested the hypothesis that the JSRV and ENTV Env proteins cause cancer by inhibiting the tumor suppressor activity of Hyal2. Consistent with this hypothesis, we show that human Hyal2 and other Hyal2 orthologs that can promote virus entry, including rat Hyal2, can suppress transformation by the Env proteins of JSRV and ENTV. Furthermore, we provide direct evidence for binding of the surface (SU) region of JSRV Env to human and rat Hyal2. However, mouse Hyal2 did not mediate entry of virions bearing JSRV or ENTV Env proteins, bound JSRV SU poorly if at all, and did not suppress transformation by the JSRV or ENTV Env proteins, indicating that mouse Hyal2 plays no role in transformation of mouse fibroblasts and that the Env proteins can transform at least some cells by a Hyal2-independent mechanism. Expression of human Hyal2 in mouse cells expressing JSRV Env caused a marked reduction in Env protein levels, indicating that human Hyal2 suppresses Env-mediated transformation in mouse cells by increasing Env degradation rather than by exerting a more general Env-independent tumor suppressor activity.
Figures

Similar articles
-
Envelope-induced cell transformation by ovine betaretroviruses.J Virol. 2002 Jun;76(11):5387-94. doi: 10.1128/jvi.76.11.5387-5394.2002. J Virol. 2002. PMID: 11991967 Free PMC article.
-
Single residues in the surface subunits of oncogenic sheep retrovirus envelopes distinguish receptor-mediated triggering for fusion at low pH and infection.Virology. 2011 Dec 20;421(2):173-83. doi: 10.1016/j.virol.2011.09.022. Epub 2011 Oct 20. Virology. 2011. PMID: 22018783
-
Enzootic nasal tumor virus envelope requires a very acidic pH for fusion activation and infection.J Virol. 2008 Sep;82(18):9023-34. doi: 10.1128/JVI.00648-08. Epub 2008 Jul 16. J Virol. 2008. PMID: 18632865 Free PMC article.
-
Hyaluronidase 2 and its intriguing role as a cell-entry receptor for oncogenic sheep retroviruses.Semin Cancer Biol. 2008 Aug;18(4):296-301. doi: 10.1016/j.semcancer.2008.03.010. Epub 2008 Mar 26. Semin Cancer Biol. 2008. PMID: 18485731 Free PMC article. Review.
-
Identification of Hyal2 as the cell-surface receptor for jaagsiekte sheep retrovirus and ovine nasal adenocarcinoma virus.Curr Top Microbiol Immunol. 2003;275:179-99. doi: 10.1007/978-3-642-55638-8_7. Curr Top Microbiol Immunol. 2003. PMID: 12596899 Review.
Cited by
-
Fusogenicity of Jaagsiekte sheep retrovirus envelope protein is dependent on low pH and is enhanced by cytoplasmic tail truncations.J Virol. 2008 Mar;82(5):2543-54. doi: 10.1128/JVI.01852-07. Epub 2007 Dec 19. J Virol. 2008. PMID: 18094165 Free PMC article.
-
Diagnosis and phylogenetic analysis of ovine pulmonary adenocarcinoma in China.Virus Genes. 2014 Feb;48(1):64-73. doi: 10.1007/s11262-013-0988-x. Epub 2013 Oct 23. Virus Genes. 2014. PMID: 24150961 Free PMC article.
-
Jaagsiekte sheep retrovirus and enzootic nasal tumor virus promoters drive gene expression in all airway epithelial cells of mice but only induce tumors in the alveolar region of the lungs.J Virol. 2011 Aug;85(15):7535-45. doi: 10.1128/JVI.00400-11. Epub 2011 May 18. J Virol. 2011. PMID: 21593165 Free PMC article.
-
Functional interaction between Env oncogene from Jaagsiekte sheep retrovirus and tumor suppressor Sprouty2.Retrovirology. 2010 Aug 2;7:62. doi: 10.1186/1742-4690-7-62. Retrovirology. 2010. PMID: 20678191 Free PMC article.
-
High levels of serum hyaluronan is an early predictor of dengue warning signs and perturbs vascular integrity.EBioMedicine. 2019 Oct;48:425-441. doi: 10.1016/j.ebiom.2019.09.014. Epub 2019 Sep 13. EBioMedicine. 2019. PMID: 31526718 Free PMC article.
References
-
- Allen, T. E., K. J. Sherrill, S. M. Crispell, M. R. Perrott, J. O. Carlson, and J. C. DeMartini. 2002. The jaagsiekte sheep retrovirus envelope gene induces transformation of the avian fibroblast cell line DF-1 but does not require a conserved SH2 binding domain. J. Gen. Virol. 83:2733-2742. - PubMed
-
- Csoka, A. B., S. W. Scherer, and R. Stern. 1999. Expression analysis of six paralogous human hyaluronidase genes clustered on chromosomes 3p21 and 7q31. Genomics 60:356-361. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

