Endoproteolytic processing of the lymphocytic choriomeningitis virus glycoprotein by the subtilase SKI-1/S1P
- PMID: 12584310
- PMCID: PMC149737
- DOI: 10.1128/jvi.77.5.2866-2872.2003
Endoproteolytic processing of the lymphocytic choriomeningitis virus glycoprotein by the subtilase SKI-1/S1P
Abstract
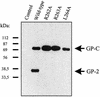
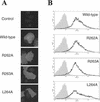
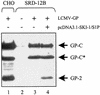
The envelope glycoprotein (GP) of lymphocytic choriomeningitis virus (LCMV) is posttranslationally cleaved into two subunits. We show here that this endoproteolytic processing is not required for transport to the cell surface but is essential for LCMV GP to mediate infectivity of pseudotyped retroviral vectors. By systematic mutational analysis of the LCMV GP cleavage site, we determined that the consensus motif R-(R/K/H)-L-(A/L/S/T/F)(265) is essential for the endoproteolytic processing. In agreement with the identified consensus motif, we show that the cellular subtilase SKI-1/S1P cleaves LCMV GP.
Figures
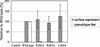


References
-
- Asper, M., P. Hofmann, C. Osmann, J. Funk, C. Metzger, M. Bruns, F. J. Kaup, H. Schmitz, and S. Gunther. 2001. First outbreak of callitrichid hepatitis in Germany: genetic characterization of the causative lymphocytic choriomeningitis virus strains. Virology 284:203-213. - PubMed
-
- Auperin, D. D., D. R. Sasso, and J. B. McCormick. 1986. Nucleotide sequence of the glycoprotein gene and intergenic region of the Lassa virus S genome RNA. Virology 154:155-167. - PubMed
-
- Basak, A., M. Chretien, and N. G. Seidah. 2002. A rapid fluorometric assay for the proteolytic activity of SKI-1/S1P based on the surface glycoprotein of the hemorrhagic fever Lassa virus. FEBS Lett. 514:333-339. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

