Transcription factor YY1 binds to the murine beta interferon promoter and regulates its transcriptional capacity with a dual activator/repressor role
- PMID: 12584314
- PMCID: PMC149748
- DOI: 10.1128/jvi.77.5.2903-2914.2003
Transcription factor YY1 binds to the murine beta interferon promoter and regulates its transcriptional capacity with a dual activator/repressor role
Abstract
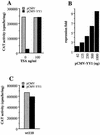
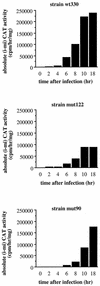
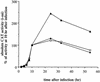
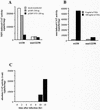
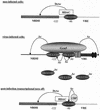
The induction of the beta interferon (IFN-beta) gene constitutes one of the first responses of the cell to virus infection. Its regulation is achieved through an intricate combination of virus-induced binding of transcription factors and local chromatin remodeling. In this work, we demonstrate that transcription factor YY1, known to interact with histone deacetylases (HDAC) and histone acetyltransferases, has a dual activator/repressor role during the regulation of the IFN-beta promoter activity. We show that YY1 specifically binds in vitro and in vivo to the murine IFN-beta promoter at positions -90 and -122. Overexpression of YY1 strongly repressed the transcriptional capacity of a stably integrated IFN-beta promoter fused to a chloramphenicol acetyltransferase reporter gene as well as the endogenous IFN activity of murine L929 cells via an HDAC activity. Stably integrated IFN-beta promoters mutated at the -90 site were no longer repressed by YY1, could no longer be activated by trichostatin A, displayed a retarded postinduction turn off, and a reduced virus-induced activity. Introduction of a mutation at the -122 site did not affect YY1-induced repression, but promoters with this mutation displayed a reduced virus-induced activity. Stably integrated full-length promoters (from position -330 to +20) mutated at both YY1-binding sites displayed extremely reduced promoter activities. We conclude that YY1 has a dual activator/repressor role on IFN-beta promoter activity depending on its binding site and time after infection.
Figures



Similar articles
-
Binding of YY1 to the proximal region of the murine beta interferon promoter is essential to allow CBP recruitment and K8H4/K14H3 acetylation on the promoter region after virus infection.Mol Cell Biol. 2006 Nov;26(22):8551-61. doi: 10.1128/MCB.00420-06. Epub 2006 Sep 5. Mol Cell Biol. 2006. PMID: 16954376 Free PMC article.
-
The nuclear factor YY1 participates in repression of the beta-casein gene promoter in mammary epithelial cells and is counteracted by mammary gland factor during lactogenic hormone induction.Mol Cell Biol. 1994 Jan;14(1):128-37. doi: 10.1128/mcb.14.1.128-137.1994. Mol Cell Biol. 1994. PMID: 8264581 Free PMC article.
-
Specific binding of high-mobility-group I (HMGI) protein and histone H1 to the upstream AT-rich region of the murine beta interferon promoter: HMGI protein acts as a potential antirepressor of the promoter.Mol Cell Biol. 1999 Apr;19(4):2803-16. doi: 10.1128/MCB.19.4.2803. Mol Cell Biol. 1999. PMID: 10082546 Free PMC article.
-
Unlocking the mechanisms of transcription factor YY1: are chromatin modifying enzymes the key?Gene. 1999 Aug 20;236(2):197-208. doi: 10.1016/s0378-1119(99)00261-9. Gene. 1999. PMID: 10452940 Review.
-
The Two Sides of YY1 in Cancer: A Friend and a Foe.Front Oncol. 2019 Nov 20;9:1230. doi: 10.3389/fonc.2019.01230. eCollection 2019. Front Oncol. 2019. PMID: 31824839 Free PMC article. Review.
Cited by
-
HDAC1/3-dependent moderate liquid-liquid phase separation of YY1 promotes METTL3 expression and AML cell proliferation.Cell Death Dis. 2022 Nov 24;13(11):992. doi: 10.1038/s41419-022-05435-y. Cell Death Dis. 2022. PMID: 36424383 Free PMC article.
-
Pregnane X receptor and yin yang 1 contribute to the differential tissue expression and induction of CYP3A5 and CYP3A4.PLoS One. 2012;7(1):e30895. doi: 10.1371/journal.pone.0030895. Epub 2012 Jan 23. PLoS One. 2012. PMID: 22292071 Free PMC article.
-
Transcription factor YY1 associates with pericentromeric gamma-satellite DNA in cycling but not in quiescent (G0) cells.Nucleic Acids Res. 2004 Aug 17;32(14):4390-9. doi: 10.1093/nar/gkh737. Print 2004. Nucleic Acids Res. 2004. PMID: 15316102 Free PMC article.
-
Exosomes originating from infection with the cytoplasmic single-stranded RNA virus Rift Valley fever virus (RVFV) protect recipient cells by inducing RIG-I mediated IFN-B response that leads to activation of autophagy.Cell Biosci. 2021 Dec 25;11(1):220. doi: 10.1186/s13578-021-00732-z. Cell Biosci. 2021. PMID: 34953502 Free PMC article.
-
Enhanceosome formation over the beta interferon promoter underlies a remote-control mechanism mediated by YY1 and YY2.Mol Cell Biol. 2005 Nov;25(22):10159-70. doi: 10.1128/MCB.25.22.10159-10170.2005. Mol Cell Biol. 2005. PMID: 16260628 Free PMC article.
References
-
- Agalioti, T., S. Lomvardas, B. Parekh, J. Yie, T. Maniatis, and D. Thanos. 2000. Ordered recruitment of chromatin modifying and general transcription factors to the IFN-β promoter. Cell 103:667-678. - PubMed
-
- Austen, M., B. Luscher, and J. M. Luscher-Firzlaff. 1997. Characterization of the transcriptional regulator YY1. J. Biol. Chem. 272:1709-1717. - PubMed
-
- Bushmeyer, S., K. Park, and M. L. Atchison. 1995. Characterization of functional domains within the multifunctional transcription factor YY1. J. Biol. Chem. 270:30213-30220. - PubMed
-
- Coull, J. J., F. Romerio, J.-M. Sun, J. L. Volker, K. M. Galvin, J. R. Davie, Y. Shi, U. Hansen, and D. M. Margolis. 2000. The human factors YY1 and LSF repress the human immunodeficiency virus type 1 long terminal repeat via recruitment of histone deacetylase 1. J. Virol. 74:6790-6799. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

