Persistent replication of hepatitis C virus replicons expressing the beta-lactamase reporter in subpopulations of highly permissive Huh7 cells
- PMID: 12584317
- PMCID: PMC149762
- DOI: 10.1128/jvi.77.5.2928-2935.2003
Persistent replication of hepatitis C virus replicons expressing the beta-lactamase reporter in subpopulations of highly permissive Huh7 cells
Abstract
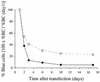
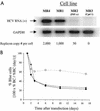
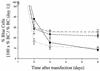
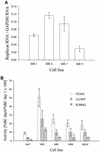
Progress toward development of better therapies for the treatment of hepatitis C virus (HCV) infection has been hampered by poor understanding of HCV biology and the lack of biological assays suitable for drug screening. Here we describe a powerful HCV replication system that employs HCV replicons expressing the beta-lactamase reporter (bla replicons) and subpopulations of Huh7 cells that are more permissive (or "enhanced") to HCV replication than naïve Huh7 cells. Enhanced cells represent a small fraction of permissive cells present among naïve Huh7 cells that is enriched during selection with replicons expressing the neomycin phosphotransferase gene (neo replicons). The level of permissiveness of cell lines harboring neo replicons can vary greatly, and the enhanced phenotype is usually revealed upon removal of the neo replicon with inhibitors of HCV replication. Replicon removal is responsible for increased permissiveness, since this effect could be reproduced either with alpha interferon or with an HCV NS5B inhibitor. Moreover, adaptive mutations present in the replicon genome used during selection do not influence the permissiveness of the resulting enhanced-cell population, suggesting that the mechanisms governing the permissiveness of enhanced cells are independent from viral adaptation. Because the beta-lactamase reporter allows simultaneous quantitation of replicon-harboring cells and reporter activity, it was possible to investigate the relationship between genome replication activity and the frequency with which transfected genomes can establish persistent replication. Our study demonstrates that differences in the replication potential of the viral genome are manifested primarily in the frequency with which persistent replication is established but modestly affect the number of replicons observed per replicon-harboring cell. Replicon copy number was found to vary over a narrow range that may be defined by a minimal number required for persistent maintenance and a maximum that is limited by the availability of essential host factors.
Figures

Similar articles
-
Dominant negative effect of wild-type NS5A on NS5A-adapted subgenomic hepatitis C virus RNA replicon.J Gen Virol. 2004 Jul;85(Pt 7):1867-1875. doi: 10.1099/vir.0.80006-0. J Gen Virol. 2004. PMID: 15218171
-
Novel hepatitis C virus reporter replicon cell lines enable efficient antiviral screening against genotype 1a.Antimicrob Agents Chemother. 2010 Aug;54(8):3099-106. doi: 10.1128/AAC.00289-10. Epub 2010 Jun 1. Antimicrob Agents Chemother. 2010. PMID: 20516274 Free PMC article.
-
Hepatitis C virus subgenomic replicons in the human embryonic kidney 293 cell line.J Virol. 2004 Jan;78(1):491-501. doi: 10.1128/jvi.78.1.491-501.2004. J Virol. 2004. PMID: 14671129 Free PMC article.
-
Replication of the hepatitis C virus in cell culture.Antiviral Res. 2003 Oct;60(2):91-102. doi: 10.1016/j.antiviral.2003.08.016. Antiviral Res. 2003. PMID: 14638404 Review.
-
[Replication of hepatitis C virus genome].Uirusu. 2008 Dec;58(2):191-8. Uirusu. 2008. PMID: 19374197 Review. Japanese.
Cited by
-
Replication fitness and NS5B drug sensitivity of diverse hepatitis C virus isolates characterized by using a transient replication assay.Antimicrob Agents Chemother. 2005 May;49(5):2059-69. doi: 10.1128/AAC.49.5.2059-2069.2005. Antimicrob Agents Chemother. 2005. PMID: 15855532 Free PMC article.
-
HCV Replicon Systems: Workhorses of Drug Discovery and Resistance.Front Cell Infect Microbiol. 2020 Jun 30;10:325. doi: 10.3389/fcimb.2020.00325. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32714881 Free PMC article. Review.
-
Effect of cell growth on hepatitis C virus (HCV) replication and a mechanism of cell confluence-based inhibition of HCV RNA and protein expression.J Virol. 2006 Feb;80(3):1181-90. doi: 10.1128/JVI.80.3.1181-1190.2006. J Virol. 2006. PMID: 16414995 Free PMC article.
-
Replication and IRES-dependent translation are both affected by core coding sequences in subgenomic GB virus B replicons.J Virol. 2003 Jul;77(13):7502-9. doi: 10.1128/jvi.77.13.7502-7509.2003. J Virol. 2003. PMID: 12805450 Free PMC article.
-
Development of a cell-based high-throughput specificity screen using a hepatitis C virus-bovine viral diarrhea virus dual replicon assay.Antimicrob Agents Chemother. 2005 Apr;49(4):1346-53. doi: 10.1128/AAC.49.4.1346-1353.2005. Antimicrob Agents Chemother. 2005. PMID: 15793110 Free PMC article.
References
-
- Blight, K. J., A. A. Kolykhalov, and C. M. Rice. 2000. Efficient initiation of HCV RNA replication in cell culture. Science 290:1972-1974. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

