An umbraviral protein, involved in long-distance RNA movement, binds viral RNA and forms unique, protective ribonucleoprotein complexes
- PMID: 12584328
- PMCID: PMC149777
- DOI: 10.1128/jvi.77.5.3031-3040.2003
An umbraviral protein, involved in long-distance RNA movement, binds viral RNA and forms unique, protective ribonucleoprotein complexes
Abstract
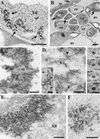
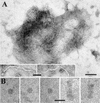
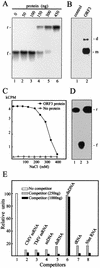
Umbraviruses are different from most other viruses in that they do not encode a conventional capsid protein (CP); therefore, no recognizable virus particles are formed in infected plants. Their lack of a CP is compensated for by the ORF3 protein, which fulfils functions that are provided by the CPs of other viruses, such as protection and long-distance movement of viral RNA. When the Groundnut rosette virus (GRV) ORF3 protein was expressed from Tobacco mosaic virus (TMV) in place of the TMV CP [TMV(ORF3)], in infected cells it interacted with the TMV RNA to form filamentous ribonucleoprotein (RNP) particles that had elements of helical structure but were not as uniform as classical virions. These RNP particles were observed in amorphous inclusions in the cytoplasm, where they were embedded within an electron-dense matrix material. The inclusions were detected in all types of cells and were abundant in phloem-associated cells, in particular companion cells and immature sieve elements. RNP-containing complexes similar in appearance to the inclusions were isolated from plants infected with TMV(ORF3) or with GRV itself. In vitro, the ORF3 protein formed oligomers and bound RNA in a manner consistent with its role in the formation of RNP complexes. It is suggested that the cytoplasmic RNP complexes formed by the ORF3 protein serve to protect viral RNA and may be the form in which it moves through the phloem. Thus, the RNP particles detected here represent a novel structure which may be used by umbraviruses as an alternative to classical virions.
Figures



Similar articles
-
Identification of a nuclear localization signal and nuclear export signal of the umbraviral long-distance RNA movement protein.J Gen Virol. 2004 May;85(Pt 5):1329-1333. doi: 10.1099/vir.0.79854-0. J Gen Virol. 2004. PMID: 15105550
-
Umbravirus-encoded proteins both stabilize heterologous viral RNA and mediate its systemic movement in some plant species.Virology. 2001 Sep 30;288(2):391-400. doi: 10.1006/viro.2001.1078. Virology. 2001. PMID: 11601910
-
Phosphorylation of tobacco mosaic virus movement protein abolishes its translation repressing ability.Virology. 1999 Aug 15;261(1):20-4. doi: 10.1006/viro.1999.9842. Virology. 1999. PMID: 10441552
-
Molecular biology of umbraviruses: phantom warriors.J Gen Virol. 2003 Aug;84(Pt 8):1951-1960. doi: 10.1099/vir.0.19219-0. J Gen Virol. 2003. PMID: 12867625 Review.
-
Structural perspective on the formation of ribonucleoprotein complex in negative-sense single-stranded RNA viruses.Trends Microbiol. 2013 Sep;21(9):475-84. doi: 10.1016/j.tim.2013.07.006. Epub 2013 Aug 14. Trends Microbiol. 2013. PMID: 23953596 Review.
Cited by
-
Differential use of 3'CITEs by the subgenomic RNA of Pea enation mosaic virus 2.Virology. 2017 Oct;510:194-204. doi: 10.1016/j.virol.2017.07.021. Epub 2017 Jul 24. Virology. 2017. PMID: 28750323 Free PMC article.
-
Cajal bodies and their role in plant stress and disease responses.RNA Biol. 2017 Jun 3;14(6):779-790. doi: 10.1080/15476286.2016.1243650. Epub 2016 Oct 11. RNA Biol. 2017. PMID: 27726481 Free PMC article. Review.
-
Interaction of a plant virus-encoded protein with the major nucleolar protein fibrillarin is required for systemic virus infection.Proc Natl Acad Sci U S A. 2007 Jun 26;104(26):11115-20. doi: 10.1073/pnas.0704632104. Epub 2007 Jun 18. Proc Natl Acad Sci U S A. 2007. PMID: 17576925 Free PMC article.
-
Viral and cellular factors involved in Phloem transport of plant viruses.Front Plant Sci. 2013 May 24;4:154. doi: 10.3389/fpls.2013.00154. eCollection 2013. Front Plant Sci. 2013. PMID: 23745125 Free PMC article.
-
Ins and Outs of Multipartite Positive-Strand RNA Plant Viruses: Packaging versus Systemic Spread.Viruses. 2016 Aug 18;8(8):228. doi: 10.3390/v8080228. Viruses. 2016. PMID: 27548199 Free PMC article. Review.
References
-
- Canto, T., D. A. M. Prior, K.-H. Hellwald, K. J. Oparka, and P. Palukaitis. 1997. Characterization of cucumber mosaic virus. IV. Movement protein and coat protein are both essential for cell-to-cell movement of cucumber mosaic virus. Virology 237:237-248. - PubMed
-
- Carrington, J. C. 2000. RNA silencing: moving targets. Nature 408:150-151. - PubMed
-
- Demler, S. A., D. G. Rucker, and G. A. de Zoeten. 1993. The chimeric nature of the genome of pea enation mosaic virus: the independent replication of RNA-2. J. Gen. Virol. 74:1-14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

