Simian-human immunodeficiency virus SHIV89.6-induced protection against intravaginal challenge with pathogenic SIVmac239 is independent of the route of immunization and is associated with a combination of cytotoxic T-lymphocyte and alpha interferon responses
- PMID: 12584336
- PMCID: PMC149756
- DOI: 10.1128/jvi.77.5.3099-3118.2003
Simian-human immunodeficiency virus SHIV89.6-induced protection against intravaginal challenge with pathogenic SIVmac239 is independent of the route of immunization and is associated with a combination of cytotoxic T-lymphocyte and alpha interferon responses
Abstract
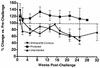
Attenuated primate lentivirus vaccines provide the most consistent protection against challenge with pathogenic simian immunodeficiency virus (SIV). Thus, they provide an excellent model to examine the influence of the route of immunization on challenge outcome and to study vaccine-induced protective anti-SIV immune responses. In the present study, rhesus macaques were immunized with live nonpathogenic simian-human immunodeficiency virus (SHIV) 89.6 either intravenously or mucosally (intranasally or intravaginally) and then challenged intravaginally with pathogenic SIVmac239. The route of immunization did not affect mucosal challenge outcome after a prolonged period of systemic infection with the nonpathogenic vaccine virus. Further, protection from the SIV challenge was associated with the induction of multiple host immune effector mechanisms. A comparison of immune responses in vaccinated-protected and vaccinated-unprotected animals revealed that vaccinated-protected animals had higher frequencies of SIV Gag-specific cytotoxic T lymphocytes and gamma interferon (IFN-gamma)-secreting cells during the acute phase postchallenge. Vaccinated-protected animals also had a more pronounced increase in peripheral blood mononuclear cell IFN-alpha mRNA levels than did the vaccinated-unprotected animals in the first few weeks after challenge. Thus, innate as well as cellular anti-SIV immune responses appeared to contribute to the SHIV89.6-induced protection against intravaginal challenge with pathogenic SIVmac239.
Figures






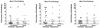
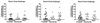
References
-
- Abel, K., M. J. Alegria-Hartman, K. Rothaeusler, M. Marthas, and C. J. Miller. 2002. The relationship between simian immunodeficiency virus RNA levels and the mRNA levels of alpha/beta interferons (IFN-α/β) and IFN-α/β-inducible Mx in lymphoid tissues of rhesus macaques during acute and chronic infection. J. Virol. 76:8433-8445. - PMC - PubMed
-
- Abel, K., M. J. Alegria-Hartman, K. Zanotto, M. B. McChesney, M. L. Marthas, and C. J. Miller. 2001. Anatomic site and immune function correlate with relative cytokine mRNA expression levels in lymphoid tissues of normal rhesus macaques. Cytokine 16:191-204. - PubMed
-
- Ahmed, R. K., C. Nilsson, Y. Wang, T. Lehner, G. Biberfeld, and R. Thorstensson. 1999. Beta-chemokine production in macaques vaccinated with live attenuated virus correlates with protection against simian immunodeficiency virus (SIVsm) challenge. J. Gen. Virol. 80:1569-1574. - PubMed
-
- Allen, T. M., B. R. Mothé, J. Sidney, P. Jing, J. L. Dzuris, M. E. Liebl, T. U. Vogel, D. H. O'Connor, X. Wang, M. C. Wussow, J. A. Thomson, J. D. Altman, D. I. Watkins, and A. Sette. 2001. CD8+ lymphocytes from simian immunodeficiency virus-infected rhesus macaques recognize 14 different epitopes bound by the major histocompatibility complex class I molecule Mamu-A*01: implications for vaccine design and testing. J. Virol. 75:738-749. - PMC - PubMed
-
- Allen, T. M., T. U. Vogel, D. H. Fuller, B. R. Mothe, S. Steffen, J. E. Boyson, T. Shipley, J. Fuller, T. Hanke, A. Sette, J. D. Altman, B. Moss, A. J. McMichael, and D. I. Watkins. 2000. Induction of AIDS virus-specific CTL activity in fresh, unstimulated peripheral blood lymphocytes from rhesus macaques vaccinated with a DNA prime/modified vaccinia virus Ankara boost regimen. J. Immunol. 164:4968-4978. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

