Ebola virus transcription activator VP30 is a zinc-binding protein
- PMID: 12584359
- PMCID: PMC149768
- DOI: 10.1128/jvi.77.5.3334-3338.2003
Ebola virus transcription activator VP30 is a zinc-binding protein
Abstract
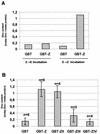
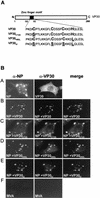
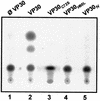
Ebola virus VP30 is an essential activator of viral transcription. In viral particles, VP30 is closely associated with the nucleocapsid complex. A conspicuous structural feature of VP30 is an unconventional zinc-binding Cys(3)-His motif comprising amino acids 68 to 95. By using a colorimetric zinc-binding assay we found that the VP30-specific Cys(3)-His motif stoichiometrically binds zinc ions in a one-to-one relationship. Substitution of the conserved cysteines and the histidine within the motif led to a complete loss of the capacity for zinc binding. Functional analyses revealed that none of the tested mutations of the proposed zinc-coordinating residues influenced binding of VP30 to nucleocapsid-like particles but, concerning its role in activating viral transcription, all resulted in a protein that was inactive.
Figures

References
-
- Buchholz, U. J., S. Finke, and K. K. Conzelmann. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 73:251-259. - PMC - PubMed
-
- DuBois, R. N., M. W. McLane, K. Ryder, L. F. Lau, and D. Nathans. 1990. A growth factor-inducible nuclear protein with a novel cysteine/histidine repetitive sequence. J. Biol. Chem. 265:19185-19191. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

