Development of murine ischemic cardiomyopathy is associated with a transient inflammatory reaction and depends on reactive oxygen species
- PMID: 12586861
- PMCID: PMC151404
- DOI: 10.1073/pnas.0438035100
Development of murine ischemic cardiomyopathy is associated with a transient inflammatory reaction and depends on reactive oxygen species
Abstract
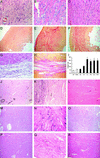
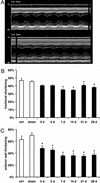
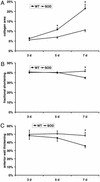
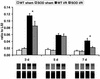
We examined the effects of daily repetitive brief (15 min) myocardial ischemia and reperfusion (I/R) in WT C57BL6 and extracellular superoxide dismutase (EC-SOD)-overexpressing mice. In the absence of myocardial necrosis, I/R resulted in persistent fibrosis in ischemic areas of C57/BL6 mice associated with persistent global and segmental anterior wall dysfunction. The I/R protocol induced chemokines (peak 3 days) followed sequentially by infiltration of macrophages and myofibroblasts (5 days). Fibrosis peaked at 7 days and was stable at 28 days despite regression of the chemokine and cellular response. Discontinuation of I/R at 7 or 28 days led to regression of fibrosis and ventricular dysfunction. In contrast, the EC-SOD mice developed markedly less chemokine induction, cell response, and fibrosis, with no ventricular dysfunction. Reversible fibrosis and ventricular dysfunction are features of human hibernating myocardium. The reduction of the cellular and functional response in EC-SOD mice suggests a role for reactive O(2) in the pathogenesis of ischemic cardiomyopathy.
Figures


References
-
- Rahimtoola S H. Circulation. 1985;72:V123–V135. - PubMed
-
- Kloner R A, Bolli R, Marban E, Reinlib L, Braunwald E. Circulation. 1998;97:1848–1867. - PubMed
-
- Vanoverschelde J L, Wijns W, Borgers M, Heyndrickx G, Depre C, Flameng W, Melin J A. Circulation. 1997;95:1961–1971. - PubMed
-
- Elsasser A, Schlepper M, Klovekorn W P, Cai W J, Zimmermann R, Muller K D, Strasser R, Kostin S, Gagel C, Munkel B, et al. Circulation. 1997;96:2920–2931. - PubMed
-
- Frangogiannis N G, Shimoni S, Chang S M, Ren G, Dewald O, Gersch C, Shan K, Aggeli C, Reardon M, Letsou G V, et al. J Am Coll Cardiol. 2002;39:1468–1474. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

