In plants, 3-o-methylglucose is phosphorylated by hexokinase but not perceived as a sugar
- PMID: 12586906
- PMCID: PMC166858
- DOI: 10.1104/pp.010538
In plants, 3-o-methylglucose is phosphorylated by hexokinase but not perceived as a sugar
Abstract
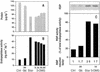
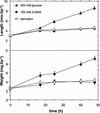
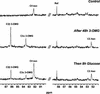
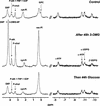
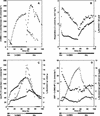
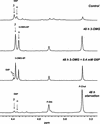
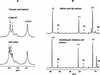
In plants, sugars are the main respiratory substrates and important signaling molecules in the regulation of carbon metabolism. Sugar signaling studies suggested that sugar sensing involves several key components, among them hexokinase (HXK). Although the sensing mechanism of HXK is unknown, several experiments support the hypothesis that hexose phosphorylation is a determining factor. Glucose (Glc) analogs transported into cells but not phosphorylated are frequently used to test this hypothesis, among them 3-O-methyl-Glc (3-OMG). The aim of the present work was to investigate the effects and fate of 3-OMG in heterotrophic plant cells. Measurements of respiration rates, protein and metabolite contents, and protease activities and amounts showed that 3-OMG is not a respiratory substrate and does not contribute to biosynthesis. Proteolysis and lipolysis are induced in 3-OMG-fed maize (Zea mays L. cv DEA) roots in the same way as in sugar-starved organs. However, contrary to the generally accepted idea, phosphorous and carbon nuclear magnetic resonance experiments and enzymatic assays prove that 3-OMG is phosphorylated to 3-OMG-6-phosphate, which accumulates in the cells. Insofar as plant HXK is involved in sugar sensing, these findings are discussed on the basis of the kinetic properties because the catalytic efficiency of HXK isolated from maize root tips is five orders of magnitude lower for 3-OMG than for Glc and Man.
Figures
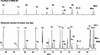
References
-
- ap Rees T. Hexose phosphate metabolism by non-photosynthetic tissues of higher plants. In: Preiss J, editor. The Biochemistry of Plants. Vol. 14. New York: Academic Press; 1988. pp. 1–33.
-
- Axelos M, Curie C, Mazzolini L, Bardet C, Lescure B. A protocol for transient gene expression in Arabidopsis thaliana protoplasts isolated from cell suspension cultures. Plant Physiol Biochem. 1992;30:123–128.
-
- Bock K, Pedersen C. Carbon-13 nuclear magnetic resonance spectroscopy of monosaccharides. In: Tipson RS, Horton D, editors. Advances in Carbohydrates Chemistry and Biochemistry. New York: Academic Press; 1983. pp. 27–66.
-
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of proteins utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

