Accumulation of mitochondrial DNA mutations in human immunodeficiency virus-infected patients treated with nucleoside-analogue reverse-transcriptase inhibitors
- PMID: 12587093
- PMCID: PMC1180231
- DOI: 10.1086/367849
Accumulation of mitochondrial DNA mutations in human immunodeficiency virus-infected patients treated with nucleoside-analogue reverse-transcriptase inhibitors
Erratum in
- Am J Hum Genet. 2003 May;72(5):1358
Abstract
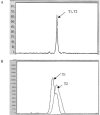
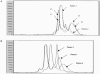
Nucleoside reverse-transcriptase inhibitor (NRTI) therapy for human immunodeficiency virus (HIV) infection has been associated with mitochondrial DNA (mtDNA) polymerase-gamma inhibition and subsequent mtDNA depletion. Effects on mtDNA mutation, although suggested by critical involvement of polymerase-gamma in DNA-repair reactions, are unknown. In the present study, we assessed the nature and frequency of mitochondrial genome sequence differences in peripheral-blood samples taken prior to NRTI therapy and after 6-77 mo of treatment in 16 NRTI-treated patients. Samples from 10 HIV-infected, treatment-naive control individuals were taken at similar time intervals. Single-stranded conformation polymorphism (SSCP) and DNA-sequencing analysis techniques were used to detect mitochondrial genome sequence variants between paired longitudinal samples, and heteroplasmic populations were quantified after cloning and repeat SSCP/sequencing. Of 16 individuals treated with NRTIs, 5 exhibited altered SSCP profiles associated with the development of novel heteroplasmic DNA sequence changes, whereas no SSCP pattern change within these regions was observed in the control individuals. Heteroplasmic sequence changes were distributed across four regions of the genome: the noncoding region to 12S ribosomal RNA, reduced-nicotinamide-adenine-dinucleotide dehydrogenase 1, and cytochrome oxidase subunits I and III. Of the total of 26 patients who were examined in the present study, 4 of 5 patients with detectable mtDNA sequence changes since commencement of therapy developed evidence of peripheral fat wasting (lipoatrophy) between sample intervals (P=.031). One patient, without detectable sequence changes on NRTI therapy, also developed lipoatrophy. Levels of mtDNA copies/cell in blood samples were determined by quantitative PCR for 11 of the 16 NRTI-exposed patients; 7 of these 11 patients showed reduced levels of mtDNA in blood after therapy, including all 3 patients tested with evidence of mtDNA sequence changes on therapy. These data indicate that NRTI therapy provides conditions permissive for the development of peripheral-blood mtDNA mutations in vivo.
Figures
References
Electronic-Database Information
-
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for the revised Cambridge reference sequence [accession number NC_001807])
References
-
- Brinkman K, ter Hofstede HJ, Burger DM, Smeitink JA, Koopmans PP (1998) Adverse effects of reverse transcriptase inhibitors: mitochondrial toxicity as common pathway. AIDS 12:1735–1744 - PubMed
-
- Hammond EL, Sayer D, Nolan D, Walker UA, de Ronde A, Montaner JSG, Cote HCF, Gahan ME, Cherry CL, Wesselingh SL, Reiss P, Mallal S. Assessment of precision and concordance of quantitative mitochondrial DNA assays: a collaborative international quality assurance study. J Clin Virol (in press) - PubMed
-
- Herrnstadt C, Elson JL, Fahy E, Preston G, Turnbull DM, Andersen C, Ghosh SS, Olefsky JM, Beal MF, Davis RE, Howell N (2002) Reduced-median-network analysis of complete mitochondrial DNA coding-region sequences for the major African, Asian, and European haplogroups. Am J Hum Genet 70:1152–1171 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

