Histone deacetylase inhibitors modulate renal disease in the MRL-lpr/lpr mouse
- PMID: 12588892
- PMCID: PMC151922
- DOI: 10.1172/JCI16153
Histone deacetylase inhibitors modulate renal disease in the MRL-lpr/lpr mouse
Abstract
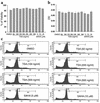
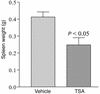
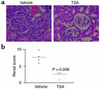
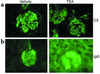
Studies in human systemic lupus erythematosus (SLE) suggest a possible role for histone deacetylases (HDACs) in skewed gene expression and disease pathogenesis. We used the MRL-lpr/lpr murine model of lupus to demonstrate that HDACs play a key role in the heightened levels of both Th1 and Th2 cytokine expression that contribute to disease. The availability of specific HDAC inhibitors (HDIs) such as trichostatin A (TSA) and suberonylanilide hydroxamic acid (SAHA) permits the study of the role of HDACs in gene regulation. Our results indicate that HDIs downregulate IL-12, IFN-gamma, IL-6, and IL-10 mRNA and protein levels in MRL-lpr/lpr splenocytes. This effect on gene transcription is associated with an increased accumulation of acetylated histones H3 and H4 in total cellular chromatin. To elucidate the in vivo effects of TSA on lupuslike disease, we treated MRL-lpr/lpr mice with TSA (0.5 mg/kg/d) for 5 weeks. Compared with vehicle-treated control mice, TSA-treated mice exhibited a significant reduction in proteinuria, glomerulonephritis, and spleen weight. Taken together, these findings suggest that increased expression of HDACs leading to an altered state of histone acetylation may be of pathologic significance in MRL-lpr/lpr mice. In addition, TSA or other HDIs may have therapeutic benefit in the treatment of SLE.
Figures







Similar articles
-
Resetting the epigenetic histone code in the MRL-lpr/lpr mouse model of lupus by histone deacetylase inhibition.J Proteome Res. 2005 Nov-Dec;4(6):2032-42. doi: 10.1021/pr050188r. J Proteome Res. 2005. PMID: 16335948
-
Aberrant expression pattern of histone acetylation modifiers and mitigation of lupus by SIRT1-siRNA in MRL/lpr mice.Scand J Rheumatol. 2009 Nov-Dec;38(6):464-71. doi: 10.3109/03009740902895750. Scand J Rheumatol. 2009. PMID: 19922023
-
Interleukin 6 (IL-6) deficiency delays lupus nephritis in MRL-Faslpr mice: the IL-6 pathway as a new therapeutic target in treatment of autoimmune kidney disease in systemic lupus erythematosus.J Rheumatol. 2010 Jan;37(1):60-70. doi: 10.3899/jrheum.090194. Epub 2009 Dec 1. J Rheumatol. 2010. PMID: 19955044
-
HDAC inhibition in lupus models.Mol Med. 2011 May-Jun;17(5-6):417-25. doi: 10.2119/molmed.2011.00055. Epub 2011 Feb 11. Mol Med. 2011. PMID: 21327298 Free PMC article. Review.
-
Nuclear localization of autoantibodies. Novel insights into protein translocation and cellular function.Ann N Y Acad Sci. 1997 Apr 5;815:263-6. doi: 10.1111/j.1749-6632.1997.tb52068.x. Ann N Y Acad Sci. 1997. PMID: 9186663 Review. No abstract available.
Cited by
-
Histone deacetylases as targets for treatment of multiple diseases.Clin Sci (Lond). 2013 Jun;124(11):651-62. doi: 10.1042/CS20120504. Clin Sci (Lond). 2013. PMID: 23414309 Free PMC article. Review.
-
Potential of epigenetic therapies in non-cancerous conditions.Front Genet. 2014 Dec 19;5:438. doi: 10.3389/fgene.2014.00438. eCollection 2014. Front Genet. 2014. PMID: 25566322 Free PMC article. Review.
-
Epigenetic histone code and autoimmunity.Clin Rev Allergy Immunol. 2010 Aug;39(1):78-84. doi: 10.1007/s12016-009-8173-7. Clin Rev Allergy Immunol. 2010. PMID: 19662539 Review.
-
Histone deacetylase inhibitor suberoylanilide hydroxamic acid reduces acute graft-versus-host disease and preserves graft-versus-leukemia effect.Proc Natl Acad Sci U S A. 2004 Mar 16;101(11):3921-6. doi: 10.1073/pnas.0400380101. Epub 2004 Mar 4. Proc Natl Acad Sci U S A. 2004. PMID: 15001702 Free PMC article.
-
Histone Deacetylase Inhibitors and Diabetic Kidney Disease.Int J Mol Sci. 2018 Sep 5;19(9):2630. doi: 10.3390/ijms19092630. Int J Mol Sci. 2018. PMID: 30189630 Free PMC article. Review.
References
-
- Gladman, D.D., and Urowitz, M.B. 1997. Prognostic subsets and mortality in systemic lupus erythematosus. In Dubois’ lupus erythematosus. D.J. Wallace and B.H. Hahn, editors. Williams & Wilkins. Baltimore, Maryland, USA. 1213–1229.
-
- Theofilopoulos AN, Dixon FJ. Murine models of systemic lupus erythematosus. Adv. Immunol. 1985;37:269–390. - PubMed
-
- Murray LJ, Lee R, Martens C. In vivo cytokine gene expression in T cell subsets of the autoimmune MRL/Mp-lpr/lpr mouse. Eur. J. Immunol. 1990;20:163–170. - PubMed
-
- Handwerger, B.S., Luzina, I., da Silva, L., Storrer, C.E., and Via, C.S. 1999. Cytokines in the immunopathogenesis of lupus. In Lupus: molecular and cellular pathogenesis. G.M. Kammer and G.C. Tsokos, editors. Humana Press. Totowa, New Jersey, USA. 321–340.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

