Developmental changes in the expression of potassium currents of embryonic, neonatal and mature mouse inner hair cells
- PMID: 12588897
- PMCID: PMC2342842
- DOI: 10.1113/jphysiol.2002.034801
Developmental changes in the expression of potassium currents of embryonic, neonatal and mature mouse inner hair cells
Erratum in
- J Physiol. 2003 Aug 1;550(Pt 3):996
Abstract
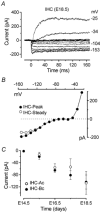
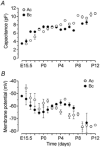
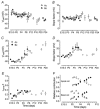
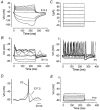
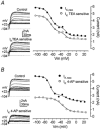
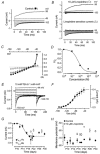
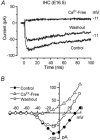
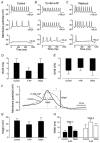
Developmental changes in electrophysiological membrane properties of mouse cochlear inner hair cells (IHCs) were studied from just after terminal differentiation up to functional maturity. As early as embryonic day 14.5 (E14.5) newly differentiated IHCs express a very small outward K+ current that is largely insensitive to 4-aminopyridine (4-AP). One day later the inward rectifier, IK1, is first observed. These immature cells initially exhibit only slow graded voltage responses under current clamp. From E17.5 spontaneous action potentials occur. During the first week of postnatal development, the outward K+ current steadily increases in size and a progressively larger fraction of the current is sensitive to 4-AP. During the second postnatal week, the activation of the 4-AP-sensitive current, by now contributing about half of the outward K+ current, shifts in the hyperpolarizing direction. Together with an increase in size of IK1, this hyperpolarizes the cell, thus inhibiting the spontaneous spike activity, although spikes could still be evoked upon depolarizing current injection. Starting at about the onset of hearing (postnatal day 12, P12) immature IHCs make the final steps towards fully functional sensory receptors with fast graded voltage responses. This is achieved mainly by the expression of the large-conductance Ca2+-activated K+ current IK,f, but also of a current indistinguishable from the negatively activating IK,n previously described in mature outer hair cells (OHCs). The 4-AP-sensitive current continues to increase after the onset of hearing to form the major part of the mature delayed rectifier, IK,s. By P20 IHCs appear mature in terms of their complement of K+ conductances.
Figures

Similar articles
-
Transient expression of an inwardly rectifying potassium conductance in developing inner and outer hair cells along the mouse cochlea.Pflugers Arch. 1999 Dec;439(1-2):113-22. doi: 10.1007/s004249900157. Pflugers Arch. 1999. PMID: 10651007
-
Resting potential and submembrane calcium concentration of inner hair cells in the isolated mouse cochlea are set by KCNQ-type potassium channels.J Neurosci. 2003 Mar 15;23(6):2141-9. doi: 10.1523/JNEUROSCI.23-06-02141.2003. J Neurosci. 2003. PMID: 12657673 Free PMC article.
-
Localization and developmental expression of BK channels in mammalian cochlear hair cells.Neuroscience. 2005;130(2):475-84. doi: 10.1016/j.neuroscience.2004.09.038. Neuroscience. 2005. PMID: 15664704
-
How to build an inner hair cell: challenges for regeneration.Hear Res. 2007 May;227(1-2):3-10. doi: 10.1016/j.heares.2006.12.005. Epub 2006 Dec 16. Hear Res. 2007. PMID: 17258412 Review.
-
Channeling your inner ear potassium: K(+) channels in vestibular hair cells.Hear Res. 2016 Aug;338:40-51. doi: 10.1016/j.heares.2016.01.015. Epub 2016 Feb 4. Hear Res. 2016. PMID: 26836968 Review.
Cited by
-
Embryonic assembly of auditory circuits: spiral ganglion and brainstem.J Physiol. 2012 May 15;590(10):2391-408. doi: 10.1113/jphysiol.2011.226886. Epub 2012 Feb 27. J Physiol. 2012. PMID: 22371481 Free PMC article.
-
Mechanisms in cochlear hair cell mechano-electrical transduction for acquisition of sound frequency and intensity.Cell Mol Life Sci. 2021 Jun;78(12):5083-5094. doi: 10.1007/s00018-021-03840-8. Epub 2021 Apr 19. Cell Mol Life Sci. 2021. PMID: 33871677 Free PMC article. Review.
-
Assessment of the expression and role of the α1-nAChR subunit in efferent cholinergic function during the development of the mammalian cochlea.J Neurophysiol. 2016 Aug 1;116(2):479-92. doi: 10.1152/jn.01038.2015. Epub 2016 Apr 20. J Neurophysiol. 2016. PMID: 27098031 Free PMC article.
-
Thyroid hormone deficiency affects postnatal spiking activity and expression of Ca2+ and K+ channels in rodent inner hair cells.J Neurosci. 2007 Mar 21;27(12):3174-86. doi: 10.1523/JNEUROSCI.3965-06.2007. J Neurosci. 2007. PMID: 17376979 Free PMC article.
-
Elementary properties of CaV1.3 Ca(2+) channels expressed in mouse cochlear inner hair cells.J Physiol. 2010 Jan 1;588(Pt 1):187-99. doi: 10.1113/jphysiol.2009.181917. Epub 2009 Nov 16. J Physiol. 2010. PMID: 19917569 Free PMC article.
References
-
- Anniko M. Cytodifferentiation of cochlear hair cells. Am J Otolaryngol. 1983;4:375–388. - PubMed
-
- Beisel KW, Nelson NC, Delimont DC, Fritzsch B. Longitudinal gradients of KCNQ4 expression in spiral ganglion and cochlear hair cells correlate with progressive hearing loss in DFNA2. Mol Brain Res. 2000;82:137–149. - PubMed
-
- Ehret G. Masked auditory thresholds, critical ratios, and scales of the basilar membrane of the housemouse (Mus musculus) J Comp Physiol. 1975;103:329–341.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

