The C terminus of initiation factor 4E-binding protein 1 contains multiple regulatory features that influence its function and phosphorylation
- PMID: 12588975
- PMCID: PMC151707
- DOI: 10.1128/MCB.23.5.1546-1557.2003
The C terminus of initiation factor 4E-binding protein 1 contains multiple regulatory features that influence its function and phosphorylation
Abstract
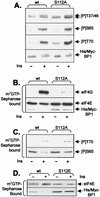
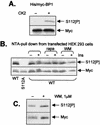
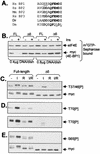
Eukaryotic initiation factor 4E (eIF4E) binds the mRNA cap structure and forms eIF4F complexes that recruit 40S subunits to the mRNA. Formation of eIF4F is blocked by eIF4E-binding proteins such as 4E-BP1, which interacts with eIF4E via a motif in the center of its 118-residue sequence. 4E-BP1 plays key roles in cell proliferation, growth, and survival. Binding of 4E-BP1 to eIF4E is regulated by hierarchical multisite phosphorylation. Here we demonstrate that three different features in the C terminus of 4E-BP1 play distinct roles in regulating its phosphorylation and function. Firstly, we identify a new phosphorylation site in its C terminus (S101). A serine or glutamate at this position is required for efficient phosphorylation at Ser65. A second C-terminal site, S112, directly affects binding of 4E-BP1 to eIF4E without influencing phosphorylation of other sites. Thirdly, a conserved C-terminal motif influences phosphorylation of multiple residues, including rapamycin-insensitive sites. These relatively long-range effects are surprising given the reportedly unstructured nature of 4E-BP1 and may imply that phosphorylation of 4E-BP1 and/or binding to eIF4E induces a more-ordered structure. 4E-BP2 and -3 lack phosphorylatable residues corresponding to both S101 and S112. However, in 4E-BP3, replacement of the alanine at the position corresponding to S112 by serine or glutamate did not confer the ability to be released from eIF4E in response to insulin.
Figures



References
-
- Avruch, J., C. Belham, Q. Weng, K. Hara, and K. Yonezawa. 2001. The p70 S6 kinase integrates nutrient and growth signals to control translational capacity. Prog. Mol. Subcell. Biol. 26:115-154. - PubMed
-
- Campbell, L. E., and C. G. Proud. 2002. Differing substrate specificities of members of the DYRK family of arginine-directed protein kinases. FEBS Lett. 510:31-36. - PubMed
-
- Chan, D. W., S. C. Son, W. Block, R. Ye, K. K. Khanna, M. S. Wold, P. Douglas, A. A. Goodarzi, J. Pelley, Y. Taya, M. F. Lavin, and S. P. Lees-Miller. 2000. Purification and characterization of ATM from human placenta. A manganese-dependent, wortmannin-sensitive serine/threonine protein kinase. J. Biol. Chem. 275:7803-7810. - PubMed
-
- Dajani, R., E. Fraser, S. M. Roe, N. Young, V. Good, T. C. Dale, and L. H. Pearl. 2001. Crystal structure of glycogen synthase kinase 3 beta: structural basis for phosphate-primed substrate specificity and autoinhibition. Cell 105:721-732. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
