Collaborative competition mechanism for gene activation in vivo
- PMID: 12588982
- PMCID: PMC151720
- DOI: 10.1128/MCB.23.5.1623-1632.2003
Collaborative competition mechanism for gene activation in vivo
Abstract
The mechanism by which gene regulatory proteins gain access to their DNA target sites is not known. In vitro, binding is inherently cooperative between arbitrary DNA binding proteins whose target sites are located within the same nucleosome. We refer to such competition-based cooperativity as collaborative competition. Here we show that arbitrarily chosen foreign DNA binding proteins, LexA and Tet repressor, cooperate with an adjacently binding endogenous activator protein, Gcn4, to coactivate expression of chromosomal reporter genes in Saccharomyces cerevisiae. Coactivation requires that the cooperating target sites be within a nucleosome-length distance; it leads to increased occupancy by Gcn4 at its binding site; and it requires both Gcn5 and Swi/Snf which, at an endogenous Gcn4-dependent promoter, act subsequent to Gcn4 binding. These results imply that collaborative competition contributes to gene regulation in vivo. They further imply that, even in the presence of the cell's full wild-type complement of chromatin remodeling factors, competition of regulatory proteins with histone octamer for access to regulatory target sites remains a quantitative determinant of gene expression levels. We speculate that initial target site recognition and binding may occur via spontaneous nucleosomal site exposure, with remodeling factor action required downstream to lock in higher levels of regulatory protein occupancy.
Figures


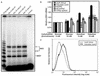

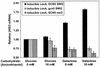
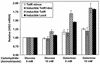
References
-
- Albrecht, G., H.-U. Mosch, B. Hoffmann, U. Reusser, and G. H. Braus. 1998. Monitoring the Gcn4 protein-mediated response in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 273:12696-12702. - PubMed
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1989. Current protocols in molecular biology. Greene Publishing Associates and Wiley-Interscience, New York, N.Y.
-
- Becker, P. B., and W. Horz. 2002. ATP-dependent nucleosome remodeling. Annu. Rev. Biochem. 71:247-273. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
