Asymmetric recognition of nonconsensus AP-1 sites by Fos-Jun and Jun-Jun influences transcriptional cooperativity with NFAT1
- PMID: 12588992
- PMCID: PMC151706
- DOI: 10.1128/MCB.23.5.1737-1749.2003
Asymmetric recognition of nonconsensus AP-1 sites by Fos-Jun and Jun-Jun influences transcriptional cooperativity with NFAT1
Abstract
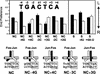
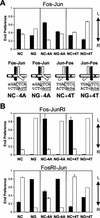
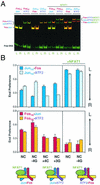
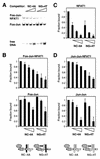
Many regulatory elements in eukaryotic promoters do not correspond to optimal recognition sequences for the transcription factors that regulate promoter function by binding to the elements. The sequence of the binding site may influence the structural and functional properties of regulatory protein complexes. Fos-Jun heterodimers were found to bind nonconsensus AP-1 sites in a preferred orientation. Oriented Fos-Jun heterodimer binding was attributed to nonidentical recognition of the two half-sites by Fos and Jun. Jun bound preferentially to the consensus half-site, whereas Fos was able to bind nonconsensus half-sites. The orientation of heterodimer binding affected the transcriptional cooperativity of Fos-Jun-NFAT1 complexes at composite regulatory elements in mammalian cells. Jun dimerization with Fos versus ATF2 caused it to bind opposite half-sites at nonconsensus AP-1 elements. Similarly, ATF2 bound to opposite half-sites in Fos-ATF2-NFAT1 and ATF2-Jun-NFAT1 complexes. The orientations of nonconsensus AP-1 sites within composite regulatory elements affected the cooperativity of Fos-Jun as well as Jun-Jun binding with NFAT1. Since Jun homodimers cannot bind to AP-1 sites in a preferred orientation, the effects of the orientations of nonconsensus AP-1 sites on the stabilities of Jun-Jun-NFAT1 complexes are likely to be due to asymmetric conformational changes in the two subunits of the homodimer. Nonconsensus AP-1 site orientation also affected the synergy of transcription activation between Jun homodimers and NFAT1 at composite regulatory elements. The asymmetric recognition of nonconsensus AP-1 sites can therefore influence the transcriptional activities of Fos and Jun both through effects on the orientation of heterodimer binding and through differential conformational changes in the two subunits of the dimer.
Figures

Similar articles
-
Dynamics of Fos-Jun-NFAT1 complexes.Proc Natl Acad Sci U S A. 2001 Apr 24;98(9):4893-8. doi: 10.1073/pnas.091095998. Proc Natl Acad Sci U S A. 2001. PMID: 11320240 Free PMC article.
-
Control of the orientation of Fos-Jun binding and the transcriptional cooperativity of Fos-Jun-NFAT1 complexes.J Biol Chem. 2001 Jun 15;276(24):21797-808. doi: 10.1074/jbc.M101494200. Epub 2001 Mar 19. J Biol Chem. 2001. PMID: 11259418
-
Long-range electrostatic interactions influence the orientation of Fos-Jun binding at AP-1 sites.J Mol Biol. 2001 Jan 19;305(3):411-27. doi: 10.1006/jmbi.2000.4286. J Mol Biol. 2001. PMID: 11152600
-
Close encounters of many kinds: Fos-Jun interactions that mediate transcription regulatory specificity.Oncogene. 2001 Apr 30;20(19):2438-52. doi: 10.1038/sj.onc.1204385. Oncogene. 2001. PMID: 11402339 Review.
-
Distinct roles of Jun : Fos and Jun : ATF dimers in oncogenesis.Oncogene. 2001 Apr 30;20(19):2453-64. doi: 10.1038/sj.onc.1204239. Oncogene. 2001. PMID: 11402340 Review.
Cited by
-
Opposing roles of FoxP1 and Nfat3 in transcriptional control of cardiomyocyte hypertrophy.Mol Cell Biol. 2011 Jul;31(14):3068-80. doi: 10.1128/MCB.00925-10. Epub 2011 May 23. Mol Cell Biol. 2011. Retraction in: Mol Cell Biol. 2020 Dec 21;41(1):e00544-20. doi: 10.1128/MCB.00544-20. PMID: 21606195 Free PMC article. Retracted.
-
Human papillomavirus type 8 E2 protein unravels JunB/Fra-1 as an activator of the beta4-integrin gene in human keratinocytes.J Virol. 2010 Feb;84(3):1376-86. doi: 10.1128/JVI.01220-09. Epub 2009 Nov 18. J Virol. 2010. PMID: 19923172 Free PMC article.
-
AP-1 and NF-κB synergize to transcriptionally activate latent HIV upon T-cell receptor activation.FEBS Lett. 2021 Mar;595(5):577-594. doi: 10.1002/1873-3468.14033. Epub 2021 Feb 9. FEBS Lett. 2021. PMID: 33421101 Free PMC article.
-
Affinity selection of DNA-binding protein complexes using mRNA display.Nucleic Acids Res. 2006 Feb 14;34(3):e27. doi: 10.1093/nar/gnj025. Nucleic Acids Res. 2006. PMID: 16478713 Free PMC article.
-
CpG-methylation regulates a class of Epstein-Barr virus promoters.PLoS Pathog. 2010 Sep 23;6(9):e1001114. doi: 10.1371/journal.ppat.1001114. PLoS Pathog. 2010. PMID: 20886097 Free PMC article.
References
-
- Abdel-Hafiz, H. A., L. E. Heasley, J. M. Kyriakis, J. Avruch, D. J. Kroll, G. L. Johnson, and J. P. Hoeffler. 1992. Activating transcription factor-2 DNA-binding activity is stimulated by phosphorylation catalyzed by p42 and p54 microtubule-associated protein kinases. Mol. Endocrinol. 6:2079-2089. - PubMed
-
- Agarwal, S., O. Avni, and A. Rao. 2000. Cell-type-restricted binding of the transcription factor NFAT to a distal IL-4 enhancer in vivo. Immunity 12:643-652. - PubMed
-
- Chen, L., J. N. Glover, P. G. Hogan, A. Rao, and S. C. Harrison. 1998. Structure of the DNA-binding domains from NFAT, Fos and Jun bound specifically to DNA. Nature 392:42-48. - PubMed
-
- Chen, L., M. G. Oakley, J. N. Glover, J. Jain, P. B. Dervan, P. G. Hogan, A. Rao, and G. L. Verdine. 1995. Only one of the two DNA-bound orientations of AP-1 found in solution cooperates with NFATp. Curr. Biol. 5:882-889. - PubMed
-
- Chinenov, Y., and T. K. Kerppola. 2001. Close encounters of many kinds: Fos-Jun interactions that mediate transcription regulatory specificity. Oncogene 20:2438-2452. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
