Rnf2 (Ring1b) deficiency causes gastrulation arrest and cell cycle inhibition
- PMID: 12589020
- PMCID: PMC151364
- DOI: 10.1073/pnas.0434312100
Rnf2 (Ring1b) deficiency causes gastrulation arrest and cell cycle inhibition
Abstract
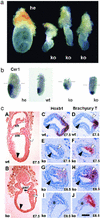
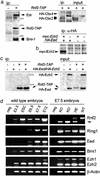
The highly homologous Rnf2 (Ring1b) and Ring1 (Ring1a) proteins were identified as in vivo interactors of the Polycomb Group (PcG) protein Bmi1. Functional ablation of Rnf2 results in gastrulation arrest, in contrast to relatively mild phenotypes in most other PcG gene null mutants belonging to the same functional group, among which is Ring1. Developmental defects occur in both embryonic and extraembryonic tissues during gastrulation. The early lethal phenotype is reminiscent of that of the PcG-gene knockouts Eed and Ezh2, which belong to a separate functional PcG group and PcG protein complex. This finding indicates that these biochemically distinct PcG complexes are both required during early mouse development. In contrast to the strong skeletal transformation in Ring1 hemizygous mice, hemizygocity for Rnf2 does not affect vertebral identity. However, it does aggravate the cerebellar phenotype in a Bmi1 null-mutant background. Together, these results suggest that Rnf2 or Ring1-containing PcG complexes have minimal functional redundancy in specific tissues, despite overlap in expression patterns. We show that the early developmental arrest in Rnf2-null embryos is partially bypassed by genetic inactivation of the Cdkn2a (Ink4aARF) locus. Importantly, this finding implicates Polycomb-mediated repression of the Cdkn2a locus in early murine development.
Figures



Similar articles
-
The polycomb group protein EED interacts with YY1, and both proteins induce neural tissue in Xenopus embryos.Mol Cell Biol. 2001 Feb;21(4):1360-9. doi: 10.1128/MCB.21.4.1360-1369.2001. Mol Cell Biol. 2001. PMID: 11158321 Free PMC article.
-
Homeotic transformations of the axial skeleton of YY1 mutant mice and genetic interaction with the Polycomb group gene Ring1/Ring1A.Mech Dev. 2006 Apr;123(4):312-20. doi: 10.1016/j.mod.2006.02.003. Epub 2006 Apr 18. Mech Dev. 2006. PMID: 16624538
-
Polycomb-group oncogenes EZH2, BMI1, and RING1 are overexpressed in prostate cancer with adverse pathologic and clinical features.Eur Urol. 2007 Aug;52(2):455-63. doi: 10.1016/j.eururo.2006.11.020. Epub 2006 Nov 17. Eur Urol. 2007. PMID: 17134822
-
Role of polycomb proteins Ring1A and Ring1B in the epigenetic regulation of gene expression.Int J Dev Biol. 2009;53(2-3):355-70. doi: 10.1387/ijdb.082690mv. Int J Dev Biol. 2009. PMID: 19412891 Review.
-
Context-dependent actions of Polycomb repressors in cancer.Oncogene. 2016 Mar 17;35(11):1341-52. doi: 10.1038/onc.2015.195. Epub 2015 Jun 8. Oncogene. 2016. PMID: 26050622 Review.
Cited by
-
Cell stemness is maintained upon concurrent expression of RB and the mitochondrial ribosomal protein S18-2.Proc Natl Acad Sci U S A. 2020 Jul 7;117(27):15673-15683. doi: 10.1073/pnas.1922535117. Epub 2020 Jun 22. Proc Natl Acad Sci U S A. 2020. PMID: 32571933 Free PMC article.
-
Recognition of H2AK119ub plays an important role in RSF1-regulated early Xenopus development.Front Cell Dev Biol. 2023 Jul 17;11:1168643. doi: 10.3389/fcell.2023.1168643. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37529237 Free PMC article.
-
An Mll4/COMPASS-Lsd1 epigenetic axis governs enhancer function and pluripotency transition in embryonic stem cells.Sci Adv. 2018 Jan 31;4(1):eaap8747. doi: 10.1126/sciadv.aap8747. eCollection 2018 Jan. Sci Adv. 2018. PMID: 29404406 Free PMC article.
-
Towards Functional Annotation of the Preimplantation Transcriptome: An RNAi Screen in Mammalian Embryos.Sci Rep. 2016 Nov 21;6:37396. doi: 10.1038/srep37396. Sci Rep. 2016. PMID: 27869233 Free PMC article.
-
The evolutionary landscape of PRC1 core components in green lineage.Planta. 2016 Apr;243(4):825-46. doi: 10.1007/s00425-015-2451-9. Epub 2016 Jan 4. Planta. 2016. PMID: 26729480 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

