Integrin-mediated tyrosine phosphorylation of Shc in T cells is regulated by protein kinase C-dependent phosphorylations of Lck
- PMID: 12589038
- PMCID: PMC149976
- DOI: 10.1091/mbc.e02-07-0382
Integrin-mediated tyrosine phosphorylation of Shc in T cells is regulated by protein kinase C-dependent phosphorylations of Lck
Abstract
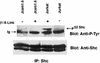
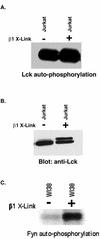
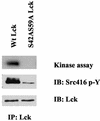
Integrin receptor signals are costimulatory for mitogenesis with the T-cell receptor during T-cell activation. A subset of integrin receptors can link to the adapter protein Shc and provide a mitogenic stimulus. Using a combination of genetic and pharmacological approaches, we show herein that integrin signaling to Shc in T cells requires the receptor tyrosine phosphatase CD45, the Src family kinase member Lck, and protein kinase C. Our results suggest a model in which integrin-dependent serine phosphorylation of Lck is the critical step that determines the efficiency of Shc tyrosine phosphorylation in T cells. Serine phosphorylation of Lck is dependent on PKC and is also linked to CD45 dephosphorylation. Mutants of Lck that cannot be phosphorylated on the critical serine residues do not signal efficiently to Shc and have greatly reduced kinase activity. This signaling from integrins to Lck may be an important step in the costimulation with the T-cell receptor during lymphocyte activation.
Figures






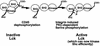
Similar articles
-
Regulation of Lck and Fyn tyrosine kinase activities by transmembrane protein tyrosine phosphatase leukocyte common antigen-related molecule.Mol Cancer Res. 2002 Dec;1(2):155-63. Mol Cancer Res. 2002. PMID: 12496362
-
Uncoupling activation-dependent HS1 phosphorylation from nuclear factor of activated T cells transcriptional activation in Jurkat T cells: differential signaling through CD3 and the costimulatory receptors CD2 and CD28.J Immunol. 1998 Nov 1;161(9):4506-12. J Immunol. 1998. PMID: 9794375
-
Roles of Lck, Syk and ZAP-70 tyrosine kinases in TCR-mediated phosphorylation of the adapter protein Shc.Eur J Immunol. 1998 Aug;28(8):2265-75. doi: 10.1002/(SICI)1521-4141(199808)28:08<2265::AID-IMMU2265>3.0.CO;2-P. Eur J Immunol. 1998. PMID: 9710204
-
The role of competing mechanisms on Lck regulation.Immunol Res. 2020 Oct;68(5):289-295. doi: 10.1007/s12026-020-09148-2. Epub 2020 Aug 14. Immunol Res. 2020. PMID: 32794043 Review.
-
The role of p56lck and p59fyn tyrosine kinases and CD45 protein tyrosine phosphatase in T-cell development and clonal selection.Immunol Rev. 1993 Oct;135:183-214. doi: 10.1111/j.1600-065x.1993.tb00649.x. Immunol Rev. 1993. PMID: 8282313 Review. No abstract available.
Cited by
-
Coordinate regulation of estrogen-mediated fibronectin matrix assembly and epidermal growth factor receptor transactivation by the G protein-coupled receptor, GPR30.Mol Endocrinol. 2009 Jul;23(7):1052-64. doi: 10.1210/me.2008-0262. Epub 2009 Apr 2. Mol Endocrinol. 2009. PMID: 19342448 Free PMC article.
-
The phosphorylation of vinculin on tyrosine residues 100 and 1065, mediated by SRC kinases, affects cell spreading.Mol Biol Cell. 2004 Sep;15(9):4234-47. doi: 10.1091/mbc.e04-03-0264. Epub 2004 Jun 30. Mol Biol Cell. 2004. PMID: 15229287 Free PMC article.
References
-
- Anderson SJ, Perlmutter RM. A signaling pathway governing early thymocyte maturation. Immunol Today. 1995;16:99–105. - PubMed
-
- Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. - PubMed
-
- Casnellie JE, Lamberts RJ. Tumor promoters cause changes in the state of phosphorylation and apparent molecular weight of a tyrosine protein kinase in T lymphocytes. J Biol Chem. 1986;261:4921–4925. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

