Reorganization of actin cytoskeleton by the phosphoinositide metabolite glycerophosphoinositol 4-phosphate
- PMID: 12589050
- PMCID: PMC149988
- DOI: 10.1091/mbc.e02-04-0179
Reorganization of actin cytoskeleton by the phosphoinositide metabolite glycerophosphoinositol 4-phosphate
Abstract
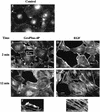
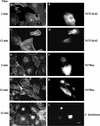
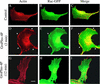
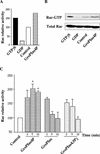
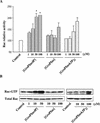
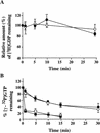
Glycerophosphoinositol 4-phosphate (GroPIns-4P) is a biologically active, water-soluble phospholipase A metabolite derived from phosphatidylinositol 4-phosphate, whose cellular concentrations have been reported to increase in Ras-transformed cells. It is therefore important to understand its biological activities. Herein, we have examined whether GroPIns-4P can regulate the organization of the actin cytoskeleton, because this could be a Ras-related function involved in cell motility and metastatic invasion. We find that in serum-starved Swiss 3T3 cells, exogenously added GroPIns-4P rapidly and potently induces the formation of membrane ruffles, and, later, the formation of stress fibers. These actin structures can be regulated by the small GTPases Cdc42, Rac, and Rho. To analyze the mechanism of action of GroPIns-4P, we selectively inactivated each of these GTPases. GroPIns-4P requires active Rac and Rho, but not Cdc42, for ruffle and stress fiber formation, respectively. Moreover, GroPIns-4P induces a rapid translocation of the green fluorescent protein-tagged Rac into ruffles, and increases the fraction of GTP-bound Rac, in intact cells. The activation of Rac by GroPIns-4P was near maximal and long-lasting. Interestingly, this feature seems to be critical in the induction of actin ruffles by GroPIns-4P.
Figures


Similar articles
-
Activation of the Rac-binding partner FHOD1 induces actin stress fibers via a ROCK-dependent mechanism.J Biol Chem. 2003 Oct 3;278(40):38902-12. doi: 10.1074/jbc.M306229200. Epub 2003 Jul 10. J Biol Chem. 2003. PMID: 12857739
-
Role of actin polymerization and adhesion to extracellular matrix in Rac- and Rho-induced cytoskeletal reorganization.J Cell Biol. 1997 Aug 25;138(4):913-26. doi: 10.1083/jcb.138.4.913. J Cell Biol. 1997. PMID: 9265656 Free PMC article.
-
Regulation of phosphorylation pathways by p21 GTPases. The p21 Ras-related Rho subfamily and its role in phosphorylation signalling pathways.Eur J Biochem. 1996 Dec 1;242(2):171-85. doi: 10.1111/j.1432-1033.1996.0171r.x. Eur J Biochem. 1996. PMID: 8973630 Review.
-
YopE of Yersinia, a GAP for Rho GTPases, selectively modulates Rac-dependent actin structures in endothelial cells.Cell Microbiol. 2001 May;3(5):301-10. doi: 10.1046/j.1462-5822.2001.00114.x. Cell Microbiol. 2001. PMID: 11298653
-
Ras-related GTPases and the cytoskeleton.Mol Biol Cell. 1992 May;3(5):475-9. doi: 10.1091/mbc.3.5.475. Mol Biol Cell. 1992. PMID: 1611153 Free PMC article. Review.
Cited by
-
A novel glycerophosphodiester phosphodiesterase, GDE5, controls skeletal muscle development via a non-enzymatic mechanism.J Biol Chem. 2010 Sep 3;285(36):27652-63. doi: 10.1074/jbc.M110.106708. Epub 2010 Jun 24. J Biol Chem. 2010. PMID: 20576599 Free PMC article.
-
The glycerophosphoinositols: cellular metabolism and biological functions.Cell Mol Life Sci. 2009 Nov;66(21):3449-67. doi: 10.1007/s00018-009-0113-4. Epub 2009 Aug 9. Cell Mol Life Sci. 2009. PMID: 19669618 Free PMC article. Review.
-
Peptide-guided targeting of GPR55 for anti-cancer therapy.Oncotarget. 2017 Jan 17;8(3):5179-5195. doi: 10.18632/oncotarget.14121. Oncotarget. 2017. PMID: 28029647 Free PMC article.
-
Faciogenital dysplasia protein (FGD1) regulates export of cargo proteins from the golgi complex via Cdc42 activation.Mol Biol Cell. 2009 May;20(9):2413-27. doi: 10.1091/mbc.e08-11-1136. Epub 2009 Mar 4. Mol Biol Cell. 2009. PMID: 19261807 Free PMC article.
-
A signalling cascade involving receptor-activated phospholipase A2, glycerophosphoinositol 4-phosphate, Shp1 and Src in the activation of cell motility.Cell Commun Signal. 2019 Mar 1;17(1):20. doi: 10.1186/s12964-019-0329-3. Cell Commun Signal. 2019. PMID: 30823936 Free PMC article.
References
-
- Alonso T, Santos E. Increased intracellular glycerophosphoinositol is a biochemical marker for transformation by membrane-associated and cytoplasmic oncogenes. Biochem Biophys Res Commun. 1990;171:14–19. - PubMed
-
- Berridge MJ. Inositol trisphosphate and calcium signaling. Nature. 1993;361:315–325. - PubMed
-
- Berrie CP, Iurisci C, Corda D. Membrane transport and in vitro metabolism of the Ras cascade messenger, glycerophosphoinositol 4-phosphate. Eur J Biochem. 1999;266:413–419. - PubMed
-
- Berrie CP, Dragani LK, van der Kaay J, Iurisci C, Brancaccio A, Rotilio D, Corda D. Maintenance of PtdIns45P2 pools under limiting inositol conditions, as assessed by liquid-chromatography-tandem mass spectrometry and PtdIns45P2 mass evaluation in Ras-transformed cells. Eur J Cancer. 2002;38:2463–2475. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

