Transforming growth factor-beta1 (TGF-beta)-induced apoptosis of prostate cancer cells involves Smad7-dependent activation of p38 by TGF-beta-activated kinase 1 and mitogen-activated protein kinase kinase 3
- PMID: 12589052
- PMCID: PMC149990
- DOI: 10.1091/mbc.02-03-0037
Transforming growth factor-beta1 (TGF-beta)-induced apoptosis of prostate cancer cells involves Smad7-dependent activation of p38 by TGF-beta-activated kinase 1 and mitogen-activated protein kinase kinase 3
Abstract
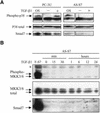
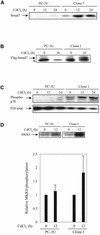
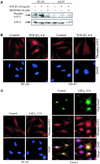
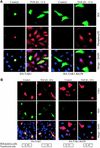
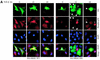
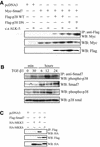
The inhibitory Smad7, a direct target gene for transforming growth factor-beta (TGF-beta), mediates TGF-beta1-induced apoptosis in several cell types. Herein, we report that apoptosis of human prostate cancer PC-3U cells induced by TGF-beta1 or Smad7 overexpression is caused by a specific activation of the p38 mitogen-activated protein kinase pathway in a TGF-beta-activated kinase 1 (TAK1)- and mitogen-activated protein kinase kinase 3 (MKK3)-dependent manner. Expression of dominant negative p38, dominant negative MKK3, or incubation with the p38 selective inhibitor [4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)1H-imidazole], prevented TGF-beta1-induced apoptosis. The expression of Smad7 was required for TGF-beta-induced activation of MKK3 and p38 kinases, and endogenous Smad7 was found to interact with phosphorylated p38 in a ligand-dependent manner. Ectopic expression of wild-type TAK1 promoted TGF-beta1-induced phosphorylation of p38 and apoptosis, whereas dominant negative TAK1 reduced TGF-beta1-induced phosphorylation of p38 and apoptosis. Endogenous Smad7 was found to interact with TAK1, and TAK1, MKK3, and p38 were coimmunoprecipitated with Smad7 in transiently transfected COS1 cells. Moreover, ectopically expressed Smad7 enhanced the coimmunoprecipitation of HA-MKK3 and Flag-p38, supporting the notion that Smad7 may act as a scaffolding protein and facilitate TAK1- and MKK3-mediated activation of p38.
Figures





References
-
- Adachi-Yamada T, Nakamura M, Irie K, Tomoyasu Y, Sano Y, Mori E, Goto S, Ueno N, Nishida Y, Matsumoto K. p38 mitogen-activated protein kinase can be involved in transforming growth factor-β superfamily signal transduction in Drosophila wing morphogenesis. Mol Cell Biol. 1999;19:2322–2329. - PMC - PubMed
-
- Afrakhte M, Morén A, Jossan S, Itoh S, Sampath K, Westermark B, Heldin C-H, Heldin N-E, ten Dijke P. Induction of inhibitory Smad6 and Smad7 mRNA by TGF-β family members. Biochem Biophys Res Commun. 1998;249:505–511. - PubMed
-
- Atfi A, Buisine M, Mazars A, Gespach C. Induction of apoptosis by DPC4, a transcriptional factor regulated by transforming growth factor-β through stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) signaling pathway. J Biol Chem. 1997a;272:24731–24734. - PubMed
-
- Atfi A, Djelloul S, Chastre E, Davis R, Gespach C. Evidence for a role of Rho-like GTPases and stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) in transforming growth factor β-mediated signaling. J Biol Chem. 1997b;272:1429–1432. - PubMed
-
- Attisano L, Wrana JL. Smads as transcriptional co-modulators. Curr Opin Cell Biol. 2000;12:235–243. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

