Raft-mediated trafficking of apical resident proteins occurs in both direct and transcytotic pathways in polarized hepatic cells: role of distinct lipid microdomains
- PMID: 12589058
- PMCID: PMC149996
- DOI: 10.1091/mbc.e02-08-0528
Raft-mediated trafficking of apical resident proteins occurs in both direct and transcytotic pathways in polarized hepatic cells: role of distinct lipid microdomains
Abstract
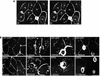
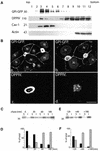
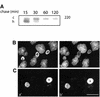
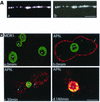
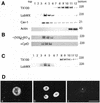
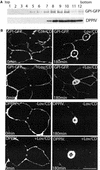
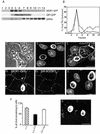
In polarized hepatic cells, pathways and molecular principles mediating the flow of resident apical bile canalicular proteins have not yet been resolved. Herein, we have investigated apical trafficking of a glycosylphosphatidylinositol-linked and two single transmembrane domain proteins on the one hand, and two polytopic proteins on the other in polarized HepG2 cells. We demonstrate that the former arrive at the bile canalicular membrane via the indirect transcytotic pathway, whereas the polytopic proteins reach the apical membrane directly, after Golgi exit. Most importantly, cholesterol-based lipid microdomains ("rafts") are operating in either pathway, and protein sorting into such domains occurs in the biosynthetic pathway, largely in the Golgi. Interestingly, rafts involved in the direct pathway are Lubrol WX insoluble but Triton X-100 soluble, whereas rafts in the indirect pathway are both Lubrol WX and Triton X-100 insoluble. Moreover, whereas cholesterol depletion alters raft-detergent insolubility in the indirect pathway without affecting apical sorting, protein missorting occurs in the direct pathway without affecting raft insolubility. The data implicate cholesterol as a traffic direction-determining parameter in the direct apical pathway. Furthermore, raft-cargo likely distinguishing single vs. multispanning membrane anchors, rather than rafts per se (co)determine the sorting pathway.
Figures


References
-
- Brown DA, Crise B, Rose JK. Mechanism of membrane anchoring affects polarized expression of two proteins in MDCK cells. Science. 1989;245:1499–1501. - PubMed
-
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. - PubMed
-
- Bull PC, Thomas GR, Rommens JM, Forbes JR, Cox DW. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet. 1993;5:327–337. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

