Treatment with bovine surfactant in severe acute respiratory distress syndrome in children: a randomized multicenter study
- PMID: 12589529
- PMCID: PMC7095123
- DOI: 10.1007/s00134-003-1650-1
Treatment with bovine surfactant in severe acute respiratory distress syndrome in children: a randomized multicenter study
Abstract
Objective: To determine whether bovine surfactant given in cases of severe pediatric acute respiratory distress syndrome (ARDS) improves oxygenation.
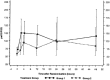
Design: Single-center study with 19 patients, followed by a multicenter randomized comparison of surfactant with a standardized treatment algorithm. Primary endpoint PaO(2)/FIO(2) at 48 h, secondary endpoints: PaO(2)/FIO(2) at 2, 4, 12, and 24 h, survival, survival without rescue, days on ventilator, subgroups analyzed by analysis of variance to identify patients who might benefit from surfactant.
Setting: Multicenter study in 19 reference centers for ARDS.
Patients: Children after the 44th postconceptional week and under 14 years old, admitted for at least 4 h, ventilated for 12-120 h, and without heart failure or chronic lung disease. In the multicenter study 35 patients were recruited; 20 were randomized to the surfactant group and 15 to the nonsurfactant group. Decreasing recruitment of patients led to a preliminary end of this study.
Interventions: Administration of 100 mg/kg bovine surfactant intratracheally under continuous ventilation and PEEP, as soon as the PaO(2)/FIO(2) ratio dropped to less than 100 for 2 h (in the pilot study increments of 50 mg/kg as long as the PaO(2)/FIO(2) did not increase by 20%). A second equivalent dose within 48 h was permitted.
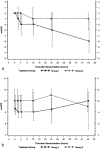
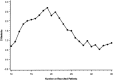
Results: In the pilot study the PaO(2)/FIO(2) increased by a mean of 100 at 48 h (n=19). A higher PaO(2)/FIO(2) ratio was observed in the surfactant group 2 h after the first dose (58 from baseline vs. 9), at 48 h there was a trend towards a higher ratio (38 from baseline vs. 22). The rate of rescue therapy was significantly lower in the surfactant group. Outcome criteria were not affected by a second surfactant dose (n=11). A significant difference in PaO(2)/FIO(2) in favor of surfactant at 48 h was found in the subgroup with an initial PaO(2)/FIO(2) ratio higher than 65 and in patients without pneumonia. CONCLUSIONS. Surfactant therapy in severe ARDS improves oxygenation immediately after administration. This improvement is sustained only in the subgroup of patients without pneumonia and that with an initial PaO(2)/FIO(2) ratio higher than 65
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

