Autoxidation of extracellular hydroquinones is a causative event for the cytotoxicity of menadione and DMNQ in A549-S cells
- PMID: 12590933
- PMCID: PMC2795776
- DOI: 10.1016/s0003-9861(02)00716-6
Autoxidation of extracellular hydroquinones is a causative event for the cytotoxicity of menadione and DMNQ in A549-S cells
Abstract
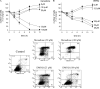
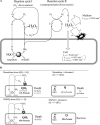
Cytotoxicity of 1,4-naphthoquinones has been attributed to intracellular reactive oxygen species (ROS) generation through one-electron-reductase-mediated redox cycling and to arylation of cellular nucleophiles. Here, however, we report that in a subclone of lung epithelial A549 cells (A549-S previously called A549-G4S (Watanabe, et al., Am. J. Physiol. 283 (2002) L726-736), the mechanism of ROS generation by menadione and by 2,3-dimethoxy-1,4-naphthoquinone (DMNQ), and therefore that of cytotoxicity, differs from the paradigm. Ninety percent of H(2)O(2) generation by both the quinones can be prevented by dicumarol, an inhibitor of NAD(P)H quinone oxidoreductase (NQO1), at the submicromolar level, regardless of the quinone concentrations. Exogenous SOD also inhibits H(2)O(2) production at low but not high concentrations of the quinones, especially DMNQ. Thus, at low quinone concentrations, superoxide-driven hydroquinone autoxidation accounts for more than half of H(2)O(2) generation by both quinones, whereas at high quinone concentrations, especially for DMNQ, comproportionation-driven hydroquinone autoxidation becomes the predominant mechanism. Hydroquinone autoxidation appears to occur predominantly in the extracellular environment than in the cytosol as extracellular catalase can dramatically attenuate quinone-induced cytotoxicity throughout the range of quinone concentrations, whereas complete inactivation of endogenous catalase or complete depletion of intracellular glutathione has only a marginal effect on their cytotoxicity. Finally, we show evidence that ROS production is a consequence of the compensatory defensive role of NQO1 against quinone arylation.
Figures






Similar articles
-
Iron deficiency aggravates DMNQ-induced cytotoxicity via redox cycling in kidney-derived cells.Free Radic Res. 2022 Jul-Aug;56(7-8):544-554. doi: 10.1080/10715762.2022.2154668. Epub 2022 Dec 16. Free Radic Res. 2022. PMID: 36469660
-
Evidence for NAD(P)H:quinone oxidoreductase 1 (NQO1)-mediated quinone-dependent redox cycling via plasma membrane electron transport: A sensitive cellular assay for NQO1.Free Radic Biol Med. 2010 Feb 1;48(3):421-9. doi: 10.1016/j.freeradbiomed.2009.11.016. Epub 2009 Nov 22. Free Radic Biol Med. 2010. PMID: 19932748
-
The relative importance of oxidative stress versus arylation in the mechanism of quinone-induced cytotoxicity to platelets.Chem Biol Interact. 1998 May 15;113(2):133-44. doi: 10.1016/s0009-2797(98)00024-6. Chem Biol Interact. 1998. PMID: 9717514
-
Quinone oxidoreductases and vitamin K metabolism.Vitam Horm. 2008;78:85-101. doi: 10.1016/S0083-6729(07)00005-2. Vitam Horm. 2008. PMID: 18374191 Review.
-
Molecular mechanisms of quinone cytotoxicity.Chem Biol Interact. 1991;80(1):1-41. doi: 10.1016/0009-2797(91)90029-7. Chem Biol Interact. 1991. PMID: 1913977 Review.
Cited by
-
Role of Human Aldo-Keto Reductases and Nuclear Factor Erythroid 2-Related Factor 2 in the Metabolic Activation of 1-Nitropyrene via Nitroreduction in Human Lung Cells.Chem Res Toxicol. 2023 Feb 20;36(2):270-280. doi: 10.1021/acs.chemrestox.2c00337. Epub 2023 Jan 24. Chem Res Toxicol. 2023. PMID: 36693016 Free PMC article.
-
Mitochondrial reserve capacity in endothelial cells: The impact of nitric oxide and reactive oxygen species.Free Radic Biol Med. 2010 Apr 1;48(7):905-14. doi: 10.1016/j.freeradbiomed.2010.01.015. Epub 2010 Jan 20. Free Radic Biol Med. 2010. PMID: 20093177 Free PMC article.
-
NAD(P)H quinone oxidoreductase 1 is essential for ozone-induced oxidative stress in mice and humans.Am J Respir Cell Mol Biol. 2009 Jul;41(1):107-13. doi: 10.1165/rcmb.2008-0381OC. Epub 2008 Dec 4. Am J Respir Cell Mol Biol. 2009. PMID: 19059883 Free PMC article.
-
The Bioenergetic Health Index is a sensitive measure of oxidative stress in human monocytes.Redox Biol. 2016 Aug;8:43-50. doi: 10.1016/j.redox.2015.12.008. Epub 2015 Dec 24. Redox Biol. 2016. PMID: 26748041 Free PMC article.
-
Contribution of reactive oxygen species to para-aminophenol toxicity in LLC-PK1 cells.Toxicol Appl Pharmacol. 2008 Jul 15;230(2):144-9. doi: 10.1016/j.taap.2008.02.014. Epub 2008 Mar 4. Toxicol Appl Pharmacol. 2008. PMID: 18396305 Free PMC article.
References
-
- Thor H, Smith MT, Hartzell P, Bellomo G, Jewell SA, Orrenius S. J. Biol. Chem. 1982;257:12419–12425. - PubMed
-
- Shi M, Gozal E, Choy HA, Forman HJ. Free Radicals Biol. Med. 1993;15:57–67. - PubMed
-
- Gant TW, Rao DN, Mason RP, Cohen GM. Chem. Biol. Interact. 1988;65:157–173. - PubMed
-
- Shi MM, Kugelman A, Iwamoto T, Tian L, Forman HJ. J. Biol. Chem. 1994;269:26512–26517. - PubMed
-
- Liu RM, Shi MM, Giulivi C, Forman HJ. Am. J. Physiol. 1998;274:L330–L336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

