Combination of single-voxel proton MR spectroscopy and apparent diffusion coefficient calculation in the evaluation of common brain tumors
- PMID: 12591638
- PMCID: PMC7974143
Combination of single-voxel proton MR spectroscopy and apparent diffusion coefficient calculation in the evaluation of common brain tumors
Abstract
Background and purpose: MR spectroscopy and apparent diffusion coefficient (ADC) calculation have been used frequently for tumor grading and differentiation during the last decade. We evaluated whether the combination of these two techniques can improve the diagnostic effectiveness of MR imaging in patients with brain tumors.
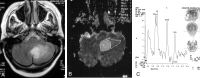
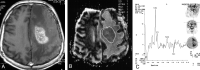
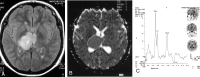
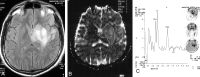
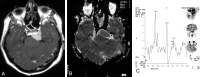
Methods: Forty-nine patients with histologically proved brain tumors (eight high- and 12 low-grade astrocytomas, eight metastases, eight nonastrocytic gliomas, seven meningiomas, three dysembryoplastic neuroepithelial tumors (DNETs), and three tuberculomas) were prospectively evaluated with contrast material-enhanced MR imaging, single-voxel proton MR spectroscopy (TE = 135 ms), and diffusion-weighted imaging (b = 0, 500, and 1000 s/mm(2)) before surgery.
Results: MR spectroscopy could differentiate benign from malignant tumors but was not useful in grading malignant tumors. In the differentiation of malignant from benign tumors, N-acetylaspartate (NAA)/choline (Cho), NAA/Cho + creatine (Cr), lactate/Cr, and alanin/Cr ratios (P <.001) were statistically more significant than NAA/Cr and lactate/lipid ratios (P <.05). Increase in lipid/Cr and alanin/Cr ratios could distinguish metastasis and meningiomas from other tumors, respectively (P <.001). DNETs could be diagnosed by their normal spectra and high ADC values (116.25 +/- 6.93 x 10(-3) mm(2)/s). Increase in lactate/Cr ratio correlated with degree of malignancy (r = -0.71). ADCs were effective for grading malignant tumors (P <.001) but not for distinguishing different tumor types with the same grade. High-grade malignant tumors (87.16 +/- 10.41 x 10(-3) mm(2)/s) had significantly lower ADC values than did low-grade malignant (115.33 +/- 11.67 x 10(-3) mm(2)/s) and benign (107.69 +/- 8.05 x 10(-3) mm(2)/s) tumors. Peritumoral ADCs were significantly higher in low-grade than in high-grade astrocytomas (P <.05).
Conclusion: Combination of calculated ADC values from tumoral core and specific relative metabolite ratios acquired by MR spectroscopy added more information to MR imaging in the differentiation and grading of brain tumors and were more useful together than each alone.
Figures
References
-
- Bruhn H, Frahm J, Gyngell ML, et al. Noninvasive differentiation of tumors with use of localized H-1 MR spectroscopy in vivo: initial experience in patients with cerebral tumors. Radiology 1989;172:541–548 - PubMed
-
- Segebarth CM, Baleriaux DF, Luyten PR, den Hollander JA. Detection of metabolic heterogeneity of human intracranial tumors in vivo by H-1 NMR spectroscopic imaging. Magn Reson Med 1990;13:62–76 - PubMed
-
- Fulham MJ, Bizzi A, Dietz MJ, et al. Mapping of brain tumor metabolites with proton MR spectroscopic imaging: clinical relevance. Radiology 1992;185:675–686 - PubMed
-
- Baker PB, Glickson JD, Brayn RN. In vivo magnetic resonance spectroscopy of human brain tumors. Top Magn Reson Imaging 1993;5:32–45 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
