Evaluation of intracoronary stenting by intravascular optical coherence tomography
- PMID: 12591841
- PMCID: PMC1767586
- DOI: 10.1136/heart.89.3.317
Evaluation of intracoronary stenting by intravascular optical coherence tomography
Abstract
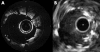
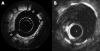
Background: Conventional contrast cineangiography and intravascular ultrasound (IVUS) provide a limited definition of vessel microstructure and are unable to evaluate dissection, tissue prolapse, and stent apposition on a size scale less than 100 micro m.
Objective: To evaluate the use of intravascular optical coherence tomography (OCT) to assess the coronary arteries in patients undergoing coronary stenting.
Methods: OCT was employed in patients having percutaneous coronary interventions. Images were obtained before initial balloon dilatation and following stent deployment, and were evaluated for vessel dissection, tissue prolapse, stent apposition, and stent asymmetry. IVUS images were obtained before OCT, using an automatic pull back device.
Results: 42 stents were imaged in 39 patients without complications. Dissection, prolapse, and incomplete stent apposition were observed more often with OCT than with IVUS. Vessel dissection was identified in eight stents by OCT and two by IVUS. Tissue prolapse was identified in 29 stents by OCT and 12 by IVUS; the extent of the prolapse (mean (SD)) was 242 (156) microm by OCT and 400 (100) microm by IVUS. Incomplete stent apposition was observed in seven stents by OCT and three by IVUS. Irregular strut separation was identified in 18 stents by both OCT and IVUS.
Conclusions: Intracoronary OCT for monitoring stent deployment is feasible and provides superior contrast and resolution of arterial pathology than IVUS.
Figures




Similar articles
-
Optical coherence tomography versus intravascular ultrasound in the evaluation of observer variability and reliability in the assessment of stent deployment: the OCTIVUS study.Catheter Cardiovasc Interv. 2015 Aug;86(2):229-35. doi: 10.1002/ccd.25854. Epub 2015 Mar 30. Catheter Cardiovasc Interv. 2015. PMID: 25620044
-
Impact of frequency-domain optical coherence tomography guidance for optimal coronary stent implantation in comparison with intravascular ultrasound guidance.Circ Cardiovasc Interv. 2012 Apr;5(2):193-201. doi: 10.1161/CIRCINTERVENTIONS.111.965111. Epub 2012 Mar 27. Circ Cardiovasc Interv. 2012. PMID: 22456026 Clinical Trial.
-
Optical coherence tomography versus intravascular ultrasound to evaluate coronary artery disease and percutaneous coronary intervention.JACC Cardiovasc Interv. 2013 Mar;6(3):228-36. doi: 10.1016/j.jcin.2012.09.017. JACC Cardiovasc Interv. 2013. PMID: 23517833
-
The Role of Intracoronary Plaque Imaging with Intravascular Ultrasound, Optical Coherence Tomography, and Near-Infrared Spectroscopy in Patients with Coronary Artery Disease.Curr Atheroscler Rep. 2016 Sep;18(9):57. doi: 10.1007/s11883-016-0607-0. Curr Atheroscler Rep. 2016. PMID: 27485540 Review.
-
Is refined OCT guidance of stent implantation needed?EuroIntervention. 2010 May;6 Suppl G:G145-53. EuroIntervention. 2010. PMID: 20542822 Review.
Cited by
-
Unrestricted utilization of frequency domain optical coherence tomography in coronary interventions.Int J Cardiovasc Imaging. 2013 Apr;29(4):741-52. doi: 10.1007/s10554-012-0135-0. Epub 2012 Oct 12. Int J Cardiovasc Imaging. 2013. PMID: 23065096
-
Natural consequence of post-intervention stent malapposition, thrombus, tissue prolapse, and dissection assessed by optical coherence tomography at mid-term follow-up.Eur Heart J Cardiovasc Imaging. 2013 Sep;14(9):865-75. doi: 10.1093/ehjci/jes299. Epub 2013 Jan 4. Eur Heart J Cardiovasc Imaging. 2013. PMID: 23291393 Free PMC article.
-
Prediction of Hemodynamic-Related Hemolysis in Carotid Stenosis and Aiding in Treatment Planning and Risk Stratification Using Computational Fluid Dynamics.Biomedicines. 2023 Dec 22;12(1):37. doi: 10.3390/biomedicines12010037. Biomedicines. 2023. PMID: 38255144 Free PMC article.
-
High-resolution three-dimensional in vivo imaging of mouse oviduct using optical coherence tomography.Biomed Opt Express. 2015 Jun 30;6(7):2713-23. doi: 10.1364/BOE.6.002713. eCollection 2015 Jul 1. Biomed Opt Express. 2015. PMID: 26203393 Free PMC article.
-
Lipid rich plaque, female gender and proximal coronary stent edge dissections.J Thromb Thrombolysis. 2013 Nov;36(4):507-13. doi: 10.1007/s11239-013-0882-3. J Thromb Thrombolysis. 2013. PMID: 23378150 Clinical Trial.
References
-
- Pasterkamp G, Wensing PJW, Post MJ, et al. Paradoxical arterial wall shrinkage may contribute to luminal narrowing of human atherosclerotic femoral arteries. Circulation 1995;91:1444–9. - PubMed
-
- Mintz GS, Kent KM, Pichard AD, et al. Contribution of inadequate arterial remodeling to the development of focal coronary artery stenoses: an intravascular ultrasound study. Circulation 1997;95:1791–8. - PubMed
-
- Colombo A, Hall P, Nakamura S, et al. Intracoronary stenting without anticoagulation accomplished with intravascular ultrasound guidance. Circulation 1995;91:1676–88. - PubMed
-
- Tearney GJ, Brezinski ME, Boppart SA, et al. Catheter-based optical imaging of a human coronary artery. Circulation 1996;94:3013. - PubMed
-
- Yabushita H, Bouma BE, Houser SL, et al. Characterization of human atherosclerosis by optical coherence tomography Circulation 2002;106:1640–5. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
