Glyceraldehyde-3-phosphate dehydrogenase has no control over glycolytic flux in Lactococcus lactis MG1363
- PMID: 12591873
- PMCID: PMC148053
- DOI: 10.1128/JB.185.5.1564-1571.2003
Glyceraldehyde-3-phosphate dehydrogenase has no control over glycolytic flux in Lactococcus lactis MG1363
Abstract
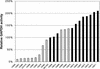
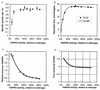
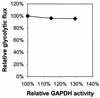
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) has previously been suggested to have almost absolute control over the glycolytic flux in Lactococcus lactis (B. Poolman, B. Bosman, J. Kiers, and W. N. Konings, J. Bacteriol. 169:5887-5890, 1987). Those studies were based on inhibitor titrations with iodoacetate, which specifically inhibits GAPDH, and the data suggested that it should be possible to increase the glycolytic flux by overproducing GAPDH activity. To test this hypothesis, we constructed a series of mutants with GAPDH activities from 14 to 210% of that of the reference strain MG1363. We found that the glycolytic flux was unchanged in the mutants overproducing GAPDH. Also, a decrease in the GAPDH activity had very little effect on the growth rate and the glycolytic flux until 25% activity was reached. Below this activity level, the glycolytic flux decreased proportionally with decreasing GAPDH activity. These data show that GAPDH activity has no control over the glycolytic flux (flux control coefficient = 0.0) at the wild-type enzyme level and that the enzyme is present in excess capacity by a factor of 3 to 4. The early experiments by Poolman and coworkers were performed with cells resuspended in buffer, i.e., nongrowing cells, and we therefore analyzed the control by GAPDH under similar conditions. We found that the glycolytic flux in resting cells was even more insensitive to changes in the GAPDH activity; in this case GAPDH was also present in a large excess and had no control over the glycolytic flux.
Figures


Similar articles
-
Glyceraldehyde-3-phosphate dehydrogenase regulation in Lactococcus lactis ssp. cremoris MG1363 or relA mutant at low pH.J Appl Microbiol. 2006 Jun;100(6):1364-72. doi: 10.1111/j.1365-2672.2006.02867.x. J Appl Microbiol. 2006. PMID: 16696685
-
Experimental determination of control of glycolysis in Lactococcus lactis.Antonie Van Leeuwenhoek. 2002 Aug;82(1-4):237-48. Antonie Van Leeuwenhoek. 2002. PMID: 12369190 Review.
-
Is the glycolytic flux in Lactococcus lactis primarily controlled by the redox charge? Kinetics of NAD(+) and NADH pools determined in vivo by 13C NMR.J Biol Chem. 2002 Aug 2;277(31):28088-98. doi: 10.1074/jbc.M202573200. Epub 2002 May 13. J Biol Chem. 2002. PMID: 12011086
-
Control of glycolysis by glyceraldehyde-3-phosphate dehydrogenase in Streptococcus cremoris and Streptococcus lactis.J Bacteriol. 1987 Dec;169(12):5887-90. doi: 10.1128/jb.169.12.5887-5890.1987. J Bacteriol. 1987. PMID: 2824452 Free PMC article.
-
Influence of Oxidative Stress on Catalytic and Non-glycolytic Functions of Glyceraldehyde-3-phosphate Dehydrogenase.Curr Med Chem. 2020;27(13):2040-2058. doi: 10.2174/0929867325666180530101057. Curr Med Chem. 2020. PMID: 29848267 Review.
Cited by
-
GAP promoter library for fine-tuning of gene expression in Pichia pastoris.Appl Environ Microbiol. 2011 Jun;77(11):3600-8. doi: 10.1128/AEM.02843-10. Epub 2011 Apr 15. Appl Environ Microbiol. 2011. PMID: 21498769 Free PMC article.
-
Overexpression of genes encoding glycolytic enzymes in Corynebacterium glutamicum enhances glucose metabolism and alanine production under oxygen deprivation conditions.Appl Environ Microbiol. 2012 Jun;78(12):4447-57. doi: 10.1128/AEM.07998-11. Epub 2012 Apr 13. Appl Environ Microbiol. 2012. PMID: 22504802 Free PMC article.
-
The pool of ADP and ATP regulates anaerobic product formation in resting cells of Lactococcus lactis.Appl Environ Microbiol. 2004 Sep;70(9):5477-84. doi: 10.1128/AEM.70.9.5477-5484.2004. Appl Environ Microbiol. 2004. PMID: 15345435 Free PMC article.
-
Proteome analyses of heme-dependent respiration in Lactococcus lactis: involvement of the proteolytic system.J Bacteriol. 2004 Mar;186(6):1648-57. doi: 10.1128/JB.186.6.1648-1657.2004. J Bacteriol. 2004. PMID: 14996795 Free PMC article.
-
Metabolic Engineering of Microorganisms to Produce Pyruvate and Derived Compounds.Molecules. 2023 Feb 2;28(3):1418. doi: 10.3390/molecules28031418. Molecules. 2023. PMID: 36771084 Free PMC article. Review.
References
-
- Andersen, H. W., M. B. Pedersen, K. Hammer, and P. R. Jensen. 2001. Lactate dehydrogenase has no control on lactate production but has a strong negative control on formate production in Lactococcus lactis. Eur. J. Biochem. 268:6379-6389. - PubMed
-
- Bakker, B. M., P. A. Michels, F. R. Opperdoes, and H. V. Westerhoff. 1999. What controls glycolysis in bloodstream form Trypanosoma brucei? J. Biol. Chem. 274:14551-14559. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

